
Dysphagia rates in single- and multiple-level anterior cervical discectomy and fusion surgery: a meta-analysis
Introduction
Cervical spine degenerative conditions effect up to two-thirds of the population and are the most common cause of acquired disability in patients over the age of 50 (1,2). These disorders commonly present with axial pain, myelopathy, radiculopathy or a combination of these symptoms. Treatment options can be classified as non-operative or operative. Non-operative treatments include analgesia and physiotherapy, and are useful primarily in cases without significant cord compression. Surgical intervention is generally indicated in with failure of conservative management or with evidence of cord compression or myelopathy (3). Factors like age, comorbidities and radiological findings are also taken into consideration when planning treatment (4,5). Multiple operative approaches have surfaced over the years with a trend changing from posterior to anterior approach particularly in 1955 when Smith and Robinson first described their technique for the anterior cervical spine approach (6,7). The optimum approach has been a source of ongoing debate and numerous studies, with remaining uncertainty about the superiority of anterior, posterior or combined techniques. Ideally, the choice of approach should be made based on patient-specific anatomic factors, the location of compressive pathology, extent of disease, imaging findings and overall medical comorbidities (3). However, the specific advantages and disadvantages of each surgical technique often complicate the management of cervical disc diseases.
Compared to the more traditional posterior approaches, anterior techniques provide favourable long-term outcomes with shortened hospital stay and reduced post-surgical pain (8). Among the anterior approaches, there are multiple widely accepted procedures performed for both single and multi-level cervical disc diseases: ACDF, anterior cervical corpectomy and fusion (ACCF), and anterior corpectomy combined with discectomy (ACCDF). These procedures provide relatively easy access to the vertebral column, yielding satisfactory surgical outcomes in most cases, and have become among the most commonly performed spinal procedures (8,9).
Despite the overall good outcomes of anterior cervical operations, there is the potential for complications including anatomical injury to the oesophagus, vertebral and carotid arteries, vagus nerve and internal jugular vein. In the early postoperative period, dysphagia is a particularly common adverse effect (10-12). In one series, Rihn et al. (13) reported postoperative dysphagia rates of 71% following anterior cervical spine surgery. The cause of dysphagia is multifactorial, and may include oesophageal denervation, haematoma formation, nerve root injury and soft tissue swelling (14-16). Where nervous injury is involved, damage occurs to the pharyngeal plexus for surgeries at spinal levels C2 to C5, the superior laryngeal nerves for surgeries at C3 to C4, the recurrent laryngeal nerves for surgeries at C5 to T1 and the hypoglossal nerve for operations above C3 (14).
Anterior approaches may be used to treat cervical degenerative diseases at up to eight cervical levels, and although several studies have reported an increase in complications such as dysphagia with multiple-level procedures (17-20) there are no meta-analyses providing high-quality evidence of this relationship. Previous meta-analysis have focused on the relationship between dysphagia and other variables such as autograft vs. allograft usage (21) and the use of Zero-profile implant systems (22). The aim of this meta-analysis was to investigate dysphagia following single-level and multiple-level anterior cervical discectomy and fusion (ACDF) surgery.
Methods
Study selection
This systematic review was conducted and reported in accordance with the preferred reporting items for systematic review and network meta-analysis checklist (PRISMA-NMA). A comprehensive search for eligible studies was conducted through the following databases: PubMed, Cochrane Central, Science Direct, and Google Scholar. We adopted the string search technique: [((Anterior AND Cervical AND Fusion) OR ACDF) AND (Dysphagia OR Swallowing disorders OR Swallowing dysfunction OR Hoarseness)], and adjusted it to each database accordingly.
Inclusion and exclusion criteria
We included all original studies that reported the rate of dysphagia as an outcome or endpoint for patients who underwent ACDF for degenerative disease, myelopathy, cervical canal stenosis, or ossification of the posterior longitudinal ligament. No restrictions on publication date and language or participant age, sex, race, place or ethnicity were applied. We excluded case reports, reviews, letters, abstract-only articles, and duplicated studies. Studies that did not report the surgical level of ACDF or reported ACDF for trauma patients were also excluded.
Data screening, extraction, and quality assessment
Two independent reviewers screened and extracted the data of the eligible studies. Extracted data included the author’s last name, year of publication, country, study design, sample number, dysphagia grading system, follow up time (endpoint), age and sex of the patients, and the number of surgical levels compared. Moreover, two independent reviewers assessed the quality of the included studies by using the Newcastle-Ottawa Quality Assessment Score for Cohort Studies (NOQAS) (23). This tool assesses three main domains in each study including the selection, comparability, and outcome with a score assigned to each and a total final score out of nine points.
Ethics approval was not required for this study given the exclusive use of previously published data.
Statistical analysis
Statistical analysis was performed using R software version 3.2.5. Due to having I2<50% and P<0.10 in the majority of the analyses, the fixed effect model was adopted. Direct comparison meta-analysis between single and multiple levels of ACDF was done and presented in the form of odds ratio (OR). Subgroup analysis was done based on the final follow up point in each study and sensitivity analysis by each time omitting one study was done to test for the robustness of the results. Funnel plot along with Egger’s test were used to test for risk of publication bias.
Results
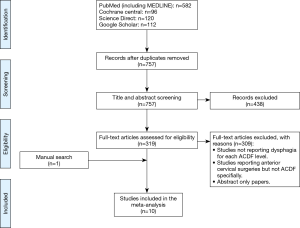
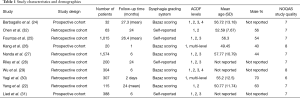
Literature search from 4 electronic databases (PubMed, Cochrane Central, Science Direct and Google Scholar) resulted 757 studies after removal of duplicates. Ten studies (22,24-32) were included for meta-analysis (Figure 1). The study characteristics and quality assessment are summarised in Table 1.
Full table
Demographics and clinical features
The 10 studies included were published from 2005 to 2017 and all were published in English. Seven studies were retrospective and 3 were prospective cohort studies. All studies reported the incidence of dysphagia following multi-level ACDF and single-level ACDF.
The 10 studies included a total of 4,018 patients (male: female ratio of 1:1.03); 1,656 patients underwent multiple-level (≥2) ACDF and 2,362 underwent single-level ACDF. The mean age ranged from 49.45 to 57.77 years.
Newcastle Ottawa Quality Assessment Scale (NOQAS) with a maximum of 9 points was applied to evaluate the quality of selection for cohort studies in terms of exposure, comparability and outcomes. Among the 10 studies, 2 studies scored 8 points, 7 studies scored 7 points and 1 study scored 6 points as shown in Table 1. Thus, the selected studies were of relatively high quality.
Single- vs. multiple-level ACDF
Mean follow-up time ranged from 2 days to 27.3 months. The follow-up period was categorised as <12, 12–24 and >24 months. Dysphagia was assessed using the Bazaz Scoring System in 6 studies (22,24,26,27,29,30), whereas the remaining 4 studies (25,28,31,32) relied on self-reported patient outcomes without a specified scale.
Of the 10 included studies, 5 reported outcomes at a follow-up period of <12 months (Figure 2A). Fixed-effect model aggregated results showed no statistical significance in dysphagia rate for patients who underwent single-level ACDF (2.8%) compared to multiple-level ACDF (3.5%) (P heterogeneity =0.509, OR =1.20, 95% CI: 0.74–1.94, I2=0%).
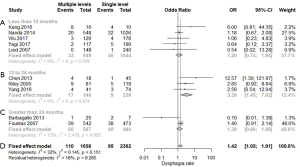
Three studies reported outcomes at a follow-up period of 12–24 months (Figure 2B). The aggregated results showed that multiple-level ACDF (12%) had a higher dysphagia rate than single-level procedures (3.7%) (P heterogeneity =0.474, OR=3.39, 95% CI: 1.45–7.92, I2=0%), with statistical significance.
Two studies with a follow-up period of >24 months (Figure 2C) showed no statistically significant difference in dysphagia rates between single-level ACDF (8.3%) and multiple-level ACDF (6.6%) (P heterogeneity =0.052, OR 1.30, 95% CI: 0.86–1.99, I2=73%).
Overall (Figure 2D), meta-analysis of the 10 studies demonstrated a higher dysphagia rate following multiple-level ACDF (6.6%) than following single-level ACDF (4.0%), a difference which was statistically significant (P heterogeneity =0.151, OR =1.42, 95% CI: 1.05–1.91, I2=32%).
Two-level vs. 1-level ACDF and 3-level vs. 1-level ACDF
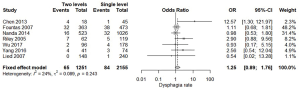
Seven studies (22,25,27-29,31,32) reported two-level and single-level ACDF outcomes, and were compared in groups based on the length of follow up (Figure 3). Fixed effect modelling demonstrated no statistically significant difference, with similar rates of dysphagia among patients treated with two-level ACDF (5.2%) and single-level ACDF (3.9%) (P heterogeneity =0.243, OR =1.25, 95% CI: 0.89–1.76, I2=24%).
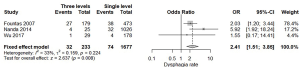
Three studies (25,27,29) reported three-level and single-level ACDF, and were compared (without grouping by length of follow-up) (Figure 4). The fixed effect model results demonstrated a higher dysphagia rate among patients treated with three-level ACDF (13.7%) compared to those treated with single-level ACDF (4.4%) (P heterogeneity =0.224, OR =2.41, 95% CI: 1.51–3.85, I2=33%). This difference was statistically significant.
Publication bias
Assessment of publication bias for all included studies was conducted using a generated Funnel plot. The Egger’s test was performed to statistically evaluate funnel plot symmetry. The results showed that no publication bias was observed.
Discussion
This systematic review and meta-analysis demonstrates higher rates of dysphagia following multiple-level ACDF than following single-level ACDF, with statistical significance particularly between 12–24 months following surgery and particularly when 3-level procedures are compared to single-level ACDF.
This data may be useful during the surgical decision-making process; dysphagia is a common and disabling complication of anterior cervical spine surgery (13,28,33-36) that is often underestimated by surgeons (25,33,36). For example, Edwards et al. (34) demonstrated that dysphagia reported in patient surveys following ACDF was underreported in the surgical records in 80% of cases.
The overall increased risk of dysphagia associated with multiple-level ACDF has been previously suggested by several studies (37-39). This relationship may be related to a variety of factors including increased operative time, increased time of oesophageal retraction, larger incisions and more extensive tissue dissection, horizontal vs. vertical incisions, greater overall trauma and larger, more intrusive spinal implants. Many of these factors have been previously shown to influence dysphagia rates. For example, in their 2012 paper Danto et al. (38) demonstrated statistically significant relationships between dysphagia and surgery duration, and between dysphagia and the incision orientation (horizontal incisions are often used for 1–2 level procedures, whereas vertical incisions may be required for fusions at 3–5 levels).
In their prospective cohort study, Lee et al. (39) demonstrated greater dysphagia rates for multiple-level ACDF than for single-level procedures, however as with our results this difference was statistically significant only at >1 year follow-up. It is possible that transient postoperative dysphagia, for example due to postoperative soft tissue swelling and oesophageal retraction injury, occurs commonly and relatively equally following both single- and multiple-level ACDF, but that long-term deficit, for example due to nerve injury, is more common after multiple-level procedures. Indeed, some authors have suggested that in the immediate postoperative period dysphagia should not be considered a complication but rather an inevitable outcome of the procedure (38,40), and this principle applies equally to single- and multiple-level surgeries. The difference in dysphagia rate over time is important when weighing the benefits and risks of a multiple-level procedure; compared to single-level ACDF, multiple-level ACDF may have a similar dysphagia risk in the immediate postoperative period but is more likely to cause long-term symptoms and therefore poses a greater risk to patient functionality and wellbeing. Our findings also emphasise the importance of long-term follow-up and monitoring particularly after multiple-level ACDF, reflecting an awareness that complications such as dysphagia may be more severe or persistent than would be expected for a single-level procedure. If identified, ongoing dysphagia can be evaluated clinically and with investigations such as plain cervical radiographs to exclude structural causes such as retropharyngeal abscesses, laryngoscopy and videofluoroscopic swallow evaluation. Treatment of long-term dysphagia depends on its severity and underlying cause, but most commonly involves behavioural measures such as dietary modification, education, postural changes and swallowing techniques. In rare cases further intervention may be required, for example with vocal cord medialisation or, in severe cases, nasogastric feeding tubes to manage aspiration risk and nutritional deficits (41).
The lack of statistical significance in our meta-analysis beyond 24 months may suggest a convergence of the dysphagia rates over the long-term as postoperative injuries continue to heal, or may reflect a lack of statistical power given that only 2 studies reported outcomes at this duration. Several studies have demonstrated low dysphagia rates at >24 months (13,37,42), and the small sample sizes may therefore limit statistical analysis. For example, in Wang et al.’s (37) multicentre retrospective study, dysphagia rate 2 years after surgery were as low as 0.4%.
The overall dysphagia rates in our meta-analysis (6.6% following multiple-level ACDF and 4.0% following single-level ACDF) are consistent with the incidence reported elsewhere in the literature, which ranges from 1.7% to 70% (13-15,28,33,34,36,42-49) The wide diversity in reported rates is likely explained by differences in study design, dysphagia reporting systems and the inherent subjectivity of self-reported outcomes (40,50,51).
Limitations
The majority of studies including in our meta-analysis were retrospective cohort studies, which inherently suffer from weaknesses related to data-collection and potential confounding exposures (52). For example, the use of treatments such as steroids may reduce postoperative inflammation and therefore limit dysphagia, however was not commonly reported and is a potential confounding factor. The ACDF surgical technique is another potentially confounding factor, in particularly the use of cervical plates compared to cages alone, and where plates are used the design and thickness of the plates. These variables have been shown to be independently significant determinants of dysphagia (53,54), however were unable to be incorporated into this meta-analysis due to inconsistent reporting and variation of surgical methods within some studies, particularly due to changes over time and between surgeons. Also, although there was no funnel plot asymmetry suggestive of publication bias according to the Egger’s test, the relatively small number of included studies may reduce the power of this measure (55). Given the retrospective design of 7 of the 10 included studies, a risk of publication bias does exist. The reliance on subjective, self-reported measurement of dysphagia (including using the Bazaz grading system) is another limitation that raises the possibility of discrepancies within and between populations. Finally, this meta-analysis solely investigated the dysphagia rates for ACDF. Further research should be carried out to determine dysphagia rates across all forms of anterior cervical surgery for consideration of comparative effectiveness.
Conclusions
Dysphagia is a common and disabling complication of ACDF, with a wide range of reported incidence in the literature ranging from 1.7% to 70% (13-15,28,33,34,36,42-49). This systematic review and meta-analysis demonstrates a statistically significant increase in complicating dysphagia following multiple-level ACDF compared to single-level ACDF at a period of 12–24 months. To improve the quality of data future studies should employ objective measures of dysphagia such as videofluoroscopic swallow evaluation and incorporate larger sample sizes with longer follow-up (56).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/jss-20-506). RM serves as the unpaid Editor-in-Chief of Journal of Spine Surgery from Sep 2015 to Sep 2025. KP serves as the unpaid Co-Editor-in-Chief Journal of Spine Surgery from Sep 2015 to Sep 2025. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fehlings MG, Arvin B. Surgical management of cervical degenerative disease: the evidence related to indications, impact, and outcome. J Neurosurg Spine 2009;11:97-100. [Crossref] [PubMed]
- Todd AG. Cervical spine: degenerative conditions. Curr Rev Musculoskelet Med 2011;4:168-74. [Crossref] [PubMed]
- Quinn JC, Kiely PD, Lebl DR, et al. Anterior surgical treatment of cervical spondylotic myelopathy: review article. HSS J 2015;11:15-25. [Crossref] [PubMed]
- Chou R, Atlas SJ, Stanos SP, et al. Nonsurgical interventional therapies for low back pain: a review of the evidence for an American Pain Society clinical practice guideline. Spine (Phila Pa 1976) 2009;34:1078-93. [Crossref] [PubMed]
- Kuijper B, Tans JT, Schimsheimer RJ, et al. Degenerative cervical radiculopathy: diagnosis and conservative treatment. A review. Eur J Neurol 2009;16:15-20. [Crossref] [PubMed]
- Denaro V, Di Martino A. Cervical spine surgery: an historical perspective. Clin Orthop Relat Res 2011;469:639-48. [Crossref] [PubMed]
- Smith GW, Robinson RA. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am 1958;40-A:607-24. [Crossref] [PubMed]
- Kim LH, D'Souza M, Ho AL, et al. Anterior Techniques in Managing Cervical Disc Disease. Cureus 2018;10:e3146. [PubMed]
- Tasiou A, Giannis T, Brotis AG, et al. Anterior cervical spine surgery-associated complications in a retrospective case-control study. J Spine Surg 2017;3:444-59. [Crossref] [PubMed]
- Joaquim AF, Murar J, Savage JW, et al. Dysphagia after anterior cervical spine surgery: a systematic review of potential preventative measures. Spine J 2014;14:2246-60. [Crossref] [PubMed]
- Boakye M, Patil CG, Santarelli J, et al. Cervical spondylotic myelopathy: complications and outcomes after spinal fusion. Neurosurgery 2008;62:455-61; discussion 461-2. [Crossref] [PubMed]
- Liu FY, Yang DL, Huang WZ, et al. Risk factors for dysphagia after anterior cervical spine surgery: A meta-analysis. Medicine 2017;96:e6267. [Crossref] [PubMed]
- Rihn JA, Kane J, Albert TJ, et al. What is the incidence and severity of dysphagia after anterior cervical surgery? Clin Orthop Relat Res 2011;469:658-65. [Crossref] [PubMed]
- Daniels AH, Riew KD, Yoo JU, et al. Adverse events associated with anterior cervical spine surgery. J Am Acad Orthop Surg 2008;16:729-38. [Crossref] [PubMed]
- Chang SW, Kakarla UK, Maughan PH, et al. Four-level anterior cervical discectomy and fusion with plate fixation: radiographic and clinical results. Neurosurgery 2010;66:639-46; discussion 646-7. [Crossref] [PubMed]
- Frempong-Boadu A, Houten JK, Osborn B, et al. Swallowing and speech dysfunction in patients undergoing anterior cervical discectomy and fusion: a prospective, objective preoperative and postoperative assessment. J Spinal Disord Tech 2002;15:362-8. [Crossref] [PubMed]
- Edwards CC 2nd, Riew KD, Anderson PA, et al. Cervical myelopathy. current diagnostic and treatment strategies. Spine J 2003;3:68-81. [Crossref] [PubMed]
- Hsu W, Dorsi MJ, Witham TF. Surgical Management of Cervical Spondylotic Myelopathy. Neurosurg Q 2009;19:302-7. [Crossref] [PubMed]
- Estcourt LJ, Desborough MJ, Doree C, et al. Plasma transfusions prior to lumbar punctures and epidural catheters for people with abnormal coagulation. Cochrane Database Syst Rev 2017;9:CD012497. [PubMed]
- Tan LA, Riew KD, Traynelis VC. Cervical Spine Deformity-Part 3: Posterior Techniques, Clinical Outcome, and Complications. Neurosurgery 2017;81:893-8. [Crossref] [PubMed]
- Shriver MF, Lewis DJ, Kshettry VR, et al. Dysphagia rates after anterior cervical diskectomy and fusion: a systematic review and meta-analysis. Global Spine J 2017;7:95-103. [Crossref] [PubMed]
- Yang Y, Ma L, Hong Y, et al. The application of Zero-profile implant in two-level and single level anterior cervical discectomy and fusion for the treatment of cervical spondylosis: a comparative study. Int J Clin Exp Med 2016;9:15667-77.
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Barbagallo GM, Romano D, Certo F, et al. Zero-P: A new zero-profile cage-plate device for single and multilevel ACDF. A single Institution series with four years maximum follow-up and review of the literature on zero-profile devices. Eur Spine J 2013;22 Suppl 6:S868-78. [Crossref] [PubMed]
- Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976) 2007;32:2310-7. [Crossref] [PubMed]
- Kang SH, Kim DK, Seo K, et al. Swallowing Function Defined by Videofluoroscopic Swallowing Studies after Anterior Cervical Discectomy and Fusion: a Prospective Study. J Korean Med Sci 2016;31:2020. [Crossref] [PubMed]
- Nanda A, Sharma M, Sonig A, et al. Surgical complications of anterior cervical diskectomy and fusion for cervical degenerative disk disease: a single surgeon's experience of 1,576 patients. World Neurosurg 2014;82:1380-7. [Crossref] [PubMed]
- Riley LH 3rd, Skolasky RL, Albert TJ, et al. Dysphagia after anterior cervical decompression and fusion: prevalence and risk factors from a longitudinal cohort study. Spine (Phila Pa 1976) 2005;30:2564-9. [Crossref] [PubMed]
- Wu B, Song F, Zhu S. Reasons of Dysphagia After Operation of Anterior Cervical Decompression and Fusion. Clin Spine Surg 2017;30:E554-9. [Crossref] [PubMed]
- Yagi K, Nakagawa H, Okazaki T, et al. Noninfectious prevertebral soft-tissue inflammation and hematoma eliciting swelling after anterior cervical discectomy and fusion. J Neurosurg Spine 2017;26:459-65. [Crossref] [PubMed]
- Lied B, Sundseth J, Helseth E. Immediate (0-6 h), early (6-72 h) and late (>72 h) complications after anterior cervical discectomy with fusion for cervical disc degeneration; discharge six hours after operation is feasible. Acta Neurochir (Wien) 2008;150:111-8; discussion 118. [Crossref] [PubMed]
- Chen Y, He Z, Yang H, et al. Anterior cervical diskectomy and fusion for adjacent segment disease. Orthopedics 2013;36:e501-8. [Crossref] [PubMed]
- Papavero L, Heese O, Klotz-Regener V, et al. The impact of esophagus retraction on early dysphagia after anterior cervical surgery: does a correlation exist? Spine (Phila Pa 1976) 2007;32:1089-93. [Crossref] [PubMed]
- Edwards CC 2nd, Karpitskaya Y, Cha C, et al. Accurate identification of adverse outcomes after cervical spine surgery. J Bone Joint Surg Am 2004;86:251-6. [Crossref] [PubMed]
- Daniels SK, Mahoney MC, Lyons GD. Persistent dysphagia and dysphonia following cervical spine surgery. Ear Nose Throat J 1998;77:470-473-5. [Crossref] [PubMed]
- Schaberg MR, Altman JI, Shapshay SM, et al. Cerebrospinal fluid leak after anterior cervical disc fusion: an unusual cause of dysphagia and neck mass. Laryngoscope 2007;117:1899-901. [Crossref] [PubMed]
- Wang T, Ma L, Yang DL, et al. Factors predicting dysphagia after anterior cervical surgery: A multicenter retrospective study for 2 years of follow-up. Medicine 2017;96:e7916. [Crossref] [PubMed]
- Danto J, DiCapua J, Nardi D, et al. Multiple cervical levels: increased risk of dysphagia and dysphonia during anterior cervical discectomy. J Neurosurg Anesthesiol 2012;24:350-5. [Crossref] [PubMed]
- Lee MJ, Bazaz R, Furey CG, et al. Risk factors for dysphagia after anterior cervical spine surgery: a two-year prospective cohort study. Spine J 2007;7:141-7. [Crossref] [PubMed]
- Campbell PG, Yadla S, Malone J, et al. Early complications related to approach in cervical spine surgery: single-center prospective study. World Neurosurg 2010;74:363-8. [Crossref] [PubMed]
- Anderson KK, Arnold PM. Oropharyngeal dysphagia after anterior cervical spine surgery: a review. Global Spine J 2013;3:273-86. [Crossref] [PubMed]
- Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery: a prospective study. Spine (Phila Pa 1976) 2002;27:2453-8. [Crossref] [PubMed]
- Yue WM, Brodner W, Highland TR. Persistent swallowing and voice problems after anterior cervical discectomy and fusion with allograft and plating: a 5- to 11-year follow-up study. Eur Spine J 2005;14:677-82. [Crossref] [PubMed]
- Winslow CP, Meyers AD. Otolaryngologic complications of the anterior approach to the cervical spine. Am J Otolaryngol 1999;20:16-27. [Crossref] [PubMed]
- Kang SH, Kim DK, Seo KM, et al. Multi-level spinal fusion and postoperative prevertebral thickness increase the risk of dysphagia after anterior cervical spine surgery. J Clin Neurosci 2011;18:1369-73. [Crossref] [PubMed]
- Chin KR, Eiszner JR, Adams SB Jr. Role of plate thickness as a cause of dysphagia after anterior cervical fusion. Spine (Phila Pa 1976) 2007;32:2585-90. [Crossref] [PubMed]
- Spennato P, Rapana A, Sannino E, et al. Retropharyngeal cerebrospinal fluid collection as a cause of postoperative dysphagia after anterior cervical discectomy. Surg Neurol 2007;67:499-503; discussion 503. [Crossref] [PubMed]
- Stewart M, Johnston RA, Stewart I, et al. Swallowing performance following anterior cervical spine surgery. Br J Neurosurg 1995;9:605-9. [Crossref] [PubMed]
- Winslow CP, Winslow TJ, Wax MK. Dysphonia and dysphagia following the anterior approach to the cervical spine. Arch Otolaryngol Head Neck Surg 2001;127:51-5. [Crossref] [PubMed]
- Vanderveldt HS, Young MF. The evaluation of dysphagia after anterior cervical spine surgery: a case report. Dysphagia 2003;18:301-4. [Crossref] [PubMed]
- Smith-Hammond CA, New KC, Pietrobon R, et al. Prospective analysis of incidence and risk factors of dysphagia in spine surgery patients: comparison of anterior cervical, posterior cervical, and lumbar procedures. Spine (Phila Pa 1976) 2004;29:1441-6. [Crossref] [PubMed]
- Euser AM, Zoccali C, Jager KJ, et al. Cohort Studies: Prospective versus Retrospective. Nephron Clin Pract 2009;113:c214-7. [Crossref] [PubMed]
- Hofstetter CP, Kesavabhotla K, Boockvar JA. Zero-profile anchored spacer reduces rate of dysphagia compared with ACDF with anterior plating. J Spinal Disord Tech 2015;28:E284-90. [Crossref] [PubMed]
- Yang H, Chen D, Wang X, et al. Zero-profile integrated plate and spacer device reduces rate of adjacent-level ossification development and dysphagia compared to ACDF with plating and cage system. Arch Orthop Trauma Surg 2015;135:781-7. [Crossref] [PubMed]
- Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- Cho SK, Lu Y, Lee DH. Dysphagia following anterior cervical spinal surgery: a systematic review. Bone Joint J 2013;95-B:868-73. [Crossref] [PubMed]


