
Surgical revision strategies for postoperative spinal implant infections (PSII)
Introduction
The number of spine surgeries has been increasing over the last decades and spinal surgery with instrumentation has grown to an essential column in the treatment for various pathologies of the spine (1,2). Due to an ageing society, the rising use of spinal implants in the young as well as in the elderly, a further increase in the incidence of instrumented spinal surgery can be expected throughout the next years (1).
As demonstrated in single studies and confirmed by meta-analysis, the additional use of intraoperative imaging and navigation has helped to further improve pedicle screw accuracy as well as reducing invasiveness and thus soft tissue injury (3) in all regions of the spine, thereby potentially reducing revision rates and additional exposure to radiation and anesthesia (4-7). Although the implementation and development of these new techniques offer faster and more minimal invasive procedures the incidence of postoperative spinal implant infections (PSII) is reported from 1% up to 20% of all instrumented spinal procedures (8,9). Furthermore early PSII as well as chronic low virulent implant associated infections have been suggested to be associated with long-term hardware failure (10-14). Hardware failure often causes loss of spinal stability, resulting in pseudarthrosis, consecutive pain as well as recurrent spinal stenosis and back pain. Extensive revision surgery to replace the loosened screws or cages, along with extension of the construct and augmentation techniques, often have to be performed in the case of symptomatic loosened hardware (14,15). Finally every single case causes additional patient morbidity affecting long-term outcome, prolonging hospitalization and of course thus raising health care costs along with loss of working days (16). Therefore the management of surgical site infection following spinal instrumentation has become an important topic in the field of spine surgery (11,17,18). So far, the level of evidence from clinical studies in the field of PSII is very limited. However critical variables for revision strategies of PSII have been identified.
Since implantable devices are highly susceptible to bacterial colonization even low virulent bacteria can cause infection and recurrent infections due to biofilms, making them difficult to detect and eradicate (19,20). In former times hardware removal was common practise in the case of deep surgical site infection across different surgical fields using implants. In instrumented spine surgery especially implant removal is discussed ambiguously due to potential loss of correction even in fused patients (21,22). Over the last years, standardized procedures for diagnostic, surgical as well as antimicrobial treatment have been developed and implemented at our institution, based on the latest recommendations in peer-reviewed literature and our own data. Here we give an overview about surgical revision strategies for PSIIs and present the key points of our protocol as well as important general surgical aspects.
Current evidence
Despite of its increasing enormous clinical and socioeconomic importance (please see above), the level of evidence regarding the best treatment strategy of PSII is quite limited. So far no data from randomized controlled trials or prospective cohort studies are available in this field (8,9,11,18,23). However, the interest and importance of the topic is evolving, and lately a number of review and overview articles analysing the clinical data available have been published (8,11,15,18,24). However, it should be noted, that since the biofilm concept, advanced microbiological techniques (e.g., Sonication, PCR), the distinction between late and early infection along with an improved understanding of the pathogenesis of infected hardware as well as new hardware and surgical techniques have evolved significantly within the last years, data from retrospective single center cohorts analysing data, collected in part over more than 10 years (and reaching back to the 90’s) are hardly comparable. Therefore beyond this data, it is certainly important to extrapolate and learn using data regarding the management from other fields of prosthetic joint infections (PJI of the hip and knee) to quickly advance in the field of spine surgery (11,20,25-27). This should incorporate clinical as well as experimental work especially in the context of biofilm and microbiological concepts, too (19,20,28-30).
Classification of PSII
It is essential classify PSII. PSII should be classified as early, delayed or late infections. This classification is important for determining the most adequate treatment regime (see Figure 1). Furthermore, specific characteristics regarding the most probable pathogen and course may be derived from the respective subgroup (25,31). The classification of PSII and the respective treatment regime has been extrapolated and modified from other implant-associated infections (20,25,32).
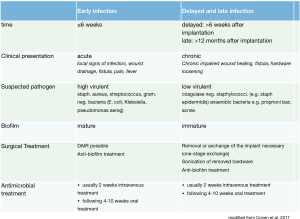
Early infections are defined as infections occurring within 6 weeks after spinal surgery with instrumentation. Patients present most notably with acute local symptoms of infection: swelling, erythema, warmth, persisting surgical site drainage and/or fistula as well as possibly systemic sings of infection like fever, increase in CRP, leukocyte cell count or erythrocyte sedimentation rate (ESR). However, especially laboratory results may be misleading as they have a low sensitivity especially with respect to low virulent pathogens, delayed PSII or in patients already receiving antibiotics (10,26,33,34). Delayed and late PSII occur more than 6 weeks after spine surgery (delayed within 1 year, late, defined as more than 1 year after surgery). Patients present with chronic wound drainage or fistula. Persisting or recurrent pain due to hardware loosing might be present as well. In delayed and late infections laboratory findings are often without pathological laboratory findings (33).
Biofilm
Implantable devices are highly susceptible to bacterial colonization and even low numbers of bacteria can cause infection. Microorganisms adhere to the implant’s surface and form biofilms (19,35). In general all bacteria are able to form a biofilm. In contrast to planktonic organisms, sessile bacteria within a mature biofilm are protected from phagocytosis, as well as other host immune responses (36). Further, as biofilm associated bacteria show an altered phenotype regarding growth rate and gene transcription they cannot be sufficiently targeted by antibiotics (28,36,37). Thus, the biofilm protects the microorganisms from the host immune system and renders them tolerant to antimicrobial treatments. Moreover, the biofilm hampers detection of the causative pathogen. Using the method of sonication, microorganisms can be released from the implants’ surface and quantitatively and qualitatively be detected from the detached biofilm in the sonication fluid. Thus to optimize detection in biofilm-associated infections, sonication of removed devices and prolonged incubation of cultures has been recommended (25). Sensitivity and specificity of sonication fluid is significantly higher compared to standard tissue cultures. Recent data showed that sonication of neurosurgical devices as well as pedicle screws is associated with a significantly higher rate of bacterial growth than in conventional cultures (14,38-40).
While in early implant-associated infections only an immature biofilm is found, in the case of delayed and late implant associated infections a mature biofilm is present. Staphylococcus aureus or gram negative bacteria are predominantly found in early infection, while the most common isolated pathogens in delayed infection are coagulase-negative staphylococci and cutibacterium acnes (formerly called propionibacterium acnes) (14,23,40,41). Moreover, it should be noted that beyond single species biofilms, as well multispecies biofilms in implant associated infection may be present (39,42). The key points including classification of PSII, suspected pathogen and the recommended procedure are summarized in Figure 1.
Hardware retention vs. hardware exchange
The effects of irrigation, debridement and implant retention (DAIR) in PSII versus hardware exchange/removal have been studied in a number of retrospective studies. Review of this studies along with the biofilm concept supports aseptic irrigation and DAIR followed by i.v. antibiotics and prolonged parenteral antibiotics as the treatment of choice for early PSII (9,11). As in early PSII an immature biofilm is present, the pathogen can be eradicated sufficiently due to its planktonic nature. Regarding the specific antibiotic regime please see the respective chapter on antibiotics.
In the case of delayed or late chronic infections a mature biofilm is present and thus hardware exchange is recommended, since the biofilm is cannot be completely eradicated from the implant’s surface. With hardware exchange or removal success rates up to 100% have been shown, while with DAIR in delayed infections recurrence rates between 20% and 50% occur (21,43-46). One study even reported a recurrence of infection in all patients with implant retention in treatment of delayed PSII. In this study cure was finally achieved after implant removal (21). As mentioned above, most of the studies are retrospective cohort studies, limiting the level of evidence. Lately the authors of a 20-year single center experience underlined the importance of implant removal/exchange in delayed infection versus DAIR in early infection (18).
Regarding exchange or removal of the interbody cage in PSII the available data is limited as well. Of course the incidence of PSII in constructs including an intervertebral cage depends on a number of different factors: stand alone cage or fusion, long/short construct, posterior, anterior or lateral approach. All of these factors impact the duration of surgery, extent of soft tissue damage, blood loss, transfusion and anaesthesia time. These factors have been identified as important procedural risk factors for PSII (8,9). Overall, the PSII incidence of an anterior or posterolateral instrumentation without a cage is lower compared to constructs including cages and posterolateral fusion (47,48). In contrast to pedicle screws and rods, removing or exchanging the interbody cage in PSII is thought to be associated with a potentially higher procedural risk due to scar tissue and the proximity to neural structures (11). So far, as available data suggests and as recently stated by an international consensus meeting on implants in infection after spine surgery, in PSII the cage can maintain if no signs of loosening along with bone loss and no signs of osteomyelitis or epidural abscess are present (11,49).
Of course, as different surfaces vary regarding their susceptibility to biofilm forming bacteria different cage materials have to be considered. However, although data from experimental work, showing lower susceptibility of titanium cages, the laboratory setting cannot be generalized for the clinical setting, as surface characteristics might also be affected by postoperative hematoma or seroma, as well as mechanical and thermal manipulation during the surgery. A number of studies have reported lower infection rates using titanium cages compared to stainless steel cages (50). Cages made of Polyethyletherketone (PEEK) have been reported to be associated with a higher infection rate compared to titanium (51). On the other hand, no difference of PEEK vs. Titanium cages was reported analysing data regarding surgical treatment of primary spinal infection (i.e., spondylodiscitis) (52,53). Taken together, the evidence level of titanium vs. PEEK is limited. In summary, as recommended by Divi et al. (11) in delayed PSII, the cage can maintain if no signs of loosening, bone loss and no signs of osteomyelitis or epidural abscess are present. Thus patients with deep PSII treated by DAIR and exchange of pedicle screws/rods and antibiotics should be followed up closely. If infection persists, cage exchange/removal should be considered if necessary using a lateral or anterior approach.
In some cases with chronic, deep spinal wound infection repeated surgical interventions might be necessary. The application of vacuum assisted closure (VAC) has been demonstrated to be an useful tool as the negative pressure promotes angiogenesis, the development of granulation tissue and reduces the number of bacteria. As recently reported VAC can efficiently and safely be applied in PSII even when the dura is exposed (54). Finally, in severe cases with significant wound defects and reduced soft tissue to cover the instrumentation and close the wound, a joint management applying VAC as well as complex flaps together with the plastic surgeons might be essential for successful treatment of PSII (55,56).
General aspects in diagnosis and treatment of PSII
As spine surgeons perform the initial surgery, of course they are as well consulted first if PSII is suspected. Most often, diagnostic measures as well as the surgical interventions are performed before microbiologists or infectologists are involved in the case. Thus, a number of important points should be considered in the management of these patients including intraoperative aspects. These key points are listed below.
- Classification of early vs. delayed PSII is indispensable for adequate management;
- Laboratory markers are insufficient to rule out PSII;
- In all patients with local signs of infection PSII has to be ruled out as soon as possible;
- Early infection (≤6 weeks) can be managed with DAIR followed by i.v. and oral antibiotics;
- In delayed/late PSII change of implants is recommended;
- If not loosened or displaced, the cage can maintain;
- All devitalized, loose or purulent material, including non incorporated bone graft should be removed until vital (bleeding) margins are obvious;
- More than 3 intraoperative tissue probes using sharp dissection should be obtained for microbiology;
- Tissue samples should be obtained, where signs of infection are most prominent;
- Microbiological samples should be obtained from the screw canal as well;
- Sonication of removed hardware is recommended;
- Antibiotics should never be started before probes for microbiology are obtained;
- Always put a drain;
- Wound closure should be performed with donati single stich suture.
A standardized interdisciplinary protocol
At our institution all patients suffering PSII are treated by an interdisciplinary team including surgeons, infectologists and microbiologists. Standardized procedures for diagnostic and surgical as well as antimicrobial treatment have been developed based on the latest recommendations in peer-reviewed literature and our own data (10,11,14,25,26,31). Protocols can be found in the PRO-IMPLANT Foundation Guidelines (https://www.pro-implant-foundation.org/). Beyond conventional microbiological methods, sonication of removed hardware in PSII has been implemented as a routine microbiological procedure at our institution. The key points of our protocol are listed in Figure 1.
Summary
PSII associated complications and revision procedures pose a tremendous socioeconomic burden. Based on the available data and the latest recommendations we have implemented an interdisciplinary protocol at our institution. The following points build the main columns of the protocol:
(I) Early diagnosis and classification of PSII; (II) in the case of early PSII—early debridement and instrument retention followed by i.v. and parenteral antibiotics; (III) in the case of delayed/late PSII—debridement and exchange of instrumentation followed by i.v. and parenteral antibiotics; (IV) sonication of removed hardware; (V) close follow up of PSII patients.
The effectiveness of the interdisciplinary standardised protocol presented in this article has been studied in a retrospective manner with respect to prosthetic joint infections (PJI) of the hip and knee, as well as in a prospective manner for infections after cranial neurosurgery. Sonication of removed hardware in PSII has been implemented as a routine microbiological procedure in PSII at our institution. The value of sonication in instrumented spinal surgery has been demonstrated (10,14,20), especially with respect to chronic, low virulent infections. Regarding PJI protocol the rate of recurrent infection was significantly reduced form 10.4% to 3.1%. Applying the cranial protocol an overall infection free survival rate of 87% (27,32) was found. We strongly believe that standardized strategies and protocols for treatment of PSII will lead to a better outcome and reduce its socioeconomic burden. Prospective randomized controlled studies are urgently needed to evaluate the optimal treatment strategy for PSII.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Matthias Pumberger) for the series “Postoperative Spinal Implant Infection” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-514). The series “Postoperative Spinal Implant Infection” was commissioned by the editorial office without any funding or sponsorship. VP is participant in the Charité Clinical Scientist Program funded by the Charité-Universitätsmedizin Berlin and the Berlin Institute of Health. The other author has no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Grotle M, Småstuen MC, Fjeld O, et al. Lumbar spine surgery across 15 years: trends, complications and reoperations in a longitudinal observational study from Norway. BMJ Open 2019;9:e028743. [Crossref] [PubMed]
- Sivasubramaniam V, Patel HC, Ozdemir BA, et al. Trends in hospital admissions and surgical procedures for degenerative lumbar spine disease in England: a 15-year time-series study. BMJ Open 2015;5:e009011. [Crossref] [PubMed]
- Vazan M, Gempt J, Meyer B, et al. Minimally invasive transforaminal lumbar interbody fusion versus open transforaminal lumbar interbody fusion: a technical description and review of the literature. Acta Neurochir (Wien) 2017;159:1137-46. [Crossref] [PubMed]
- Czabanka M, Haemmerli J, Hecht N, et al. Spinal navigation for posterior instrumentation of C1-2 instability using a mobile intraoperative CT scanner. J Neurosurg Spine 2017;27:268-75. [Crossref] [PubMed]
- Goldberg J, Bayerl SH, Witzel C, et al. Surgical workflow for fully navigated high sacral amputation in sacral chordoma. Neurosurg Rev 2020;43:343-9. [Crossref] [PubMed]
- Hecht N, Yassin H, Czabanka M, et al. Intraoperative Computed Tomography Versus 3D C-Arm Imaging for Navigated Spinal Instrumentation. Spine 2018;43:370-7. [Crossref] [PubMed]
- Rienmüller A, Buchmann N, Kirschke JS, et al. Accuracy of CT-navigated pedicle screw positioning in the cervical and upper thoracic region with and without prior anterior surgery and ventral plating. Bone Joint J 2017;99-B:1373-80. [Crossref] [PubMed]
- Parchi PD, Evangelisti G, Andreani L, et al. Postoperative Spine Infections. Orthop Rev (Pavia) 2015;7:5900. [Crossref] [PubMed]
- Lall RR, Wong AP, Lall RR, et al. Evidence-based management of deep wound infection after spinal instrumentation. J Clin Neurosci 2015;22:238-42. [Crossref] [PubMed]
- Bürger J, Akgün D, Strube P, et al. Sonication of removed implants improves microbiological diagnosis of postoperative spinal infections. Eur Spine J 2019;28:768-74. [Crossref] [PubMed]
- Divi SN, Kepler CK, Boody BS, et al. Consensus on Implants in Infections After Spine Surgery. Clin Spine Surg 2020;33:163-71. [Crossref] [PubMed]
- Fanous AA, Kolcun JPG, Brusko GD, et al. Surgical Site Infection as a Risk Factor for Long-Term Instrumentation Failure in Patients with Spinal Deformity: A Retrospective Cohort Study. World Neurosurg 2019;132:e514-9. [Crossref] [PubMed]
- Leitner L, Malaj I, Sadoghi P, et al. Pedicle screw loosening is correlated to chronic subclinical deep implant infection: a retrospective database analysis. Eur Spine J 2018;27:2529-35. [Crossref] [PubMed]
- Prinz V, Bayerl S, Renz N, et al. High frequency of low-virulent microorganisms detected by sonication of pedicle screws: a potential cause for implant failure. J Neurosurg Spine 2019;31:424-9. [Crossref] [PubMed]
- Atesok K, Vaccaro A, Stippler M, et al. Fate of Hardware in Spinal Infections. Surg Infect (Larchmt) 2020;21:404-10. [Crossref] [PubMed]
- O’Hara LM, Thom KA, Preas MA. Update to the Centers for Disease Control and Prevention and the Healthcare Infection Control Practices Advisory Committee Guideline for the Prevention of Surgical Site Infection (2017): A summary, review, and strategies for implementation. Am J Infect Control 2018;46:602-9. [Crossref] [PubMed]
- Bachy M, Bouyer B, Vialle R. Infections after spinal correction and fusion for spinal deformities in childhood and adolescence. Int Orthop 2012;36:465-9. [Crossref] [PubMed]
- Kalfas F, Severi P, Scudieri C.. Infection with Spinal Instrumentation: A 20-Year, Single-Institution Experience with Review of Pathogenesis, Diagnosis, Prevention, and Management. Asian J Neurosurg 2019;14:1181-9. [Crossref] [PubMed]
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999;284:1318-22. [Crossref] [PubMed]
- Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med 2004;351:1645-54. [Crossref] [PubMed]
- Hedequist D, Haugen A, Hresko T, et al. Failure of attempted implant retention in spinal deformity delayed surgical site infections. Spine 2009;34:60-4. [Crossref] [PubMed]
- Potter BK, Kirk KL, Shah SA, et al. Loss of coronal correction following instrumentation removal in adolescent idiopathic scoliosis. Spine 2006;31:67-72. [Crossref] [PubMed]
- Kasliwal MK, Tan LA, Traynelis VC. Infection with spinal instrumentation: Review of pathogenesis, diagnosis, prevention, and management. Surg Neurol Int 2013;4:S392-403. [Crossref] [PubMed]
- Atesok K, Papavassiliou E, Heffernan MJ, et al. Current Strategies in Prevention of Postoperative Infections in Spine Surgery. Global Spine J 2020;10:183-94. [Crossref] [PubMed]
- Conen A, Fux CA, Vajkoczy P, et al. Management of infections associated with neurosurgical implanted devices. Expert Rev Anti Infect Ther 2017;15:241-55. [Crossref] [PubMed]
- Li C, Renz N, Trampuz A, et al. Twenty common errors in the diagnosis and treatment of periprosthetic joint infection. Int Orthop 2020;44:3-14. [Crossref] [PubMed]
- Renz N, Özdirik B, Finger T, et al. Infections following cranial neurosurgery - prospective cohort of 103 episodes treated according to a standardized algorithm. World Neurosurg 2018;116:e491-9. [Crossref] [PubMed]
- Bjarnsholt T, Alhede M, Alhede M, et al. The in vivo biofilm. Trends Microbiol 2013;21:466-74. [Crossref] [PubMed]
- Crabbé A, Jensen PØ, Bjarnsholt T, et al. Antimicrobial Tolerance and Metabolic Adaptations in Microbial Biofilms. Trends Microbiol 2019;27:850-63. [Crossref] [PubMed]
- Schwarzer S, James GA, Goeres D, et al. The efficacy of topical agents used in wounds for managing chronic biofilm infections: A systematic review. J Infect 2020;80:261-70. [Crossref] [PubMed]
- Renz N, Perka C, Trampuz A. Management of periprosthetic infections of the knee. Orthopade 2016;45:65-71. [Crossref] [PubMed]
- Karczewski D, Winkler T, Renz N, et al. A standardized interdisciplinary algorithm for the treatment of prosthetic joint infections. Bone Joint J 2019;101-B:132-9. [Crossref] [PubMed]
- Akgün D, Bürger J, Pumberger M, et al. C-reactive protein misdiagnoses delayed postoperative spinal implant infections in patients with low-virulent microorganisms. Eur Spine J 2019;28:2990-5. [Crossref] [PubMed]
- Miksić NG. Spinal infections with and without hardware: the viewpoint of an infectious disease specialist. Eur J Orthop Surg Traumatol 2013;23 Suppl 1:S21-8. [Crossref] [PubMed]
- Verstraeten N, Braeken K, Debkumari B, et al. Living on a surface: swarming and biofilm formation. Trends Microbiol 2008;16:496-506. [Crossref] [PubMed]
- Hernández-Jiménez E, Del Campo R, Toledano V, et al. Biofilm vs. planktonic bacterial mode of growth: which do human macrophages prefer? Biochem Biophys Res Commun 2013;441:947-52. [Crossref] [PubMed]
- Jefferson KK. What drives bacteria to produce a biofilm? FEMS Microbiol Lett 2004;236:163-73. [Crossref] [PubMed]
- Jost GF, Wasner M, Taub E, et al. Sonication of catheter tips for improved detection of microorganisms on external ventricular drains and ventriculo-peritoneal shunts. J Clin Neurosci 2014;21:578-82. [Crossref] [PubMed]
- Prinz V, Bayerl S, Renz N, et al. Sonication Improves Pathogen Detection in Ventriculoperitoneal Shunt-Associated Infections. Neurosurgery 2019;85:516-23. [Crossref] [PubMed]
- Sampedro MF, Huddleston PM, Piper KE, et al. A biofilm approach to detect bacteria on removed spinal implants. Spine 2010;35:1218-24. [Crossref] [PubMed]
- Hahn F, Zbinden R, Min K. Late implant infections caused by Propionibacterium acnes in scoliosis surgery. Eur Spine J 2005;14:783-8. [Crossref] [PubMed]
- Røder HL, Sørensen SJ, Burmølle M. Studying Bacterial Multispecies Biofilms: Where to Start? Trends Microbiol 2016;24:503-13. [Crossref] [PubMed]
- Collins I, Wilson-MacDonald J, Chami G, et al. The diagnosis and management of infection following instrumented spinal fusion. Eur Spine J 2008;17:445-50. [Crossref] [PubMed]
- Maruo K, Berven SH. Outcome and treatment of postoperative spine surgical site infections: predictors of treatment success and failure. J Orthop Sci 2014;19:398-404. [Crossref] [PubMed]
- Richards BR, Emara KM. Delayed infections after posterior TSRH spinal instrumentation for idiopathic scoliosis: revisited. Spine 2001;26:1990-6. [Crossref] [PubMed]
- Viola RW, King HA, Adler SM, et al. Delayed infection after elective spinal instrumentation and fusion. A retrospective analysis of eight cases. Spine 1997;22:2444-50; discussion 2450-1. [Crossref] [PubMed]
- Ahn DK, Park HS, Choi DJ, et al. The difference of surgical site infection according to the methods of lumbar fusion surgery. J Spinal Disord Tech 2012;25:E230-4. [Crossref] [PubMed]
- Hee HT, Castro FP, Majd ME, et al. Anterior/posterior lumbar fusion versus transforaminal lumbar interbody fusion: analysis of complications and predictive factors. J Spinal Disord 2001;14:533-40. [Crossref] [PubMed]
- Mirovsky Y, Floman Y, Smorgick Y, et al. Management of deep wound infection after posterior lumbar interbody fusion with cages. J Spinal Disord Tech 2007;20:127-31. [Crossref] [PubMed]
- Soultanis KC, Pyrovolou N, Zahos KA, et al. Late postoperative infection following spinal instrumentation: stainless steel versus titanium implants. J Surg Orthop Adv 2008;17:193-9. [PubMed]
- Weinstein MA, McCabe JP, Cammisa FP. Postoperative spinal wound infection: a review of 2,391 consecutive index procedures. J Spinal Disord 2000;13:422-6. [Crossref] [PubMed]
- Schomacher M, Finger T, Koeppen D, et al. Application of titanium and polyetheretherketone cages in the treatment of pyogenic spondylodiscitis. Clin Neurol Neurosurg 2014;127:65-70. [Crossref] [PubMed]
- Shiban E, Janssen I, da Cunha PR, et al. Safety and efficacy of polyetheretherketone (PEEK) cages in combination with posterior pedicel screw fixation in pyogenic spinal infection. Acta Neurochir (Wien) 2016;158:1851-7. [Crossref] [PubMed]
- Lee R, Beder D, Street J, et al. The use of vacuum-assisted closure in spinal wound infections with or without exposed dura. Eur Spine J 2018;27:2536-42. [Crossref] [PubMed]
- Dumanian GA, Ondra SL, Liu J, et al. Muscle flap salvage of spine wounds with soft tissue defects or infection. Spine 2003;28:1203-11. [Crossref] [PubMed]
- Inglesby DC, Young ZT, Alshareef M, et al. Paraspinous Muscle Flaps for the Treatment of Complex Spinal Wounds. Spine (Phila Pa 1976) 2020;45:599-604. [Crossref] [PubMed]


