
Comprehensive treatment algorithm of postoperative spinal implant infection
Introduction
Since the development of pedicle screw based internal fixator (1), the number of spinal fusions has been increasing over the past decades and is nowadays one of the most applied procedures in surgery (2-4). The use of spinal internal fixation devices like screws and rods enables the treatment of a wide range of spinal diseases like traumatic and pathological fractures, degenerative diseases like osteochondrosis and spinal stenosis or deformities like primary and secondary scoliosis or hyperkyphosis. Unfortunately, the implantation of foreign devices into the spine as well as in all parts of the body, is associated with an increased risk of postoperative infections (5). Those kinds of infections are called implant-associated infections and belong to one of the most dangerous complication in orthopedic and traumatological surgery and are associated with increased mortality, prolonged hospitalization and enhanced costs for healthcare system (5,6). At the spine, postoperative infections have been reported with an incidence of up to 20% (7-12). In revision spine surgery it has been shown that postoperative spinal implant infections (PSIIs) occur in even up to 27% (13,14).
Classification and diagnosis of PSII
The classification of PSIIs is almost similar to the classification of periprosthetic joint infections and divides implant-associated infections according to time between former surgery and appearance of symptoms or duration of symptoms (acute or chronic) and the path of infection (post interventional, haematogenous or per continuitatem). The different categories of PSII typically differ in clinical presentation and the causative pathogens. A summary of the classification is shown in Table 1.

Full table
Acute or early-onset PSIIs with diagnosis within 6 weeks after the former surgery or onset of symptoms <6 weeks ago are typically characterized by classical signs of infections such as fever, wound healing disorders like redness or prolonged wound drainage, acute pain, but also acute neurological deficits (15-18). The diagnosis of acute PSII is usually made by the clinical presentation and can be supported by laboratory parameters (p.e. increased CRP or leucocyte count). The causative microorganisms of acute PSII can be detected by blood cultures (haematogenous), peri-implant tissue samples or examination of removed spinal implants. The most common microorganisms of acute PSII are Staphylococcus aureus, followed by β-hemolytic streptococci, gram-negative bacilli, and coagulase-negative staphylococci (19,20). In some rare cases of early PSII, also fungal pathogens can be found.
Chronic postoperative spinal infections occur >6 weeks after the former surgery or the symptoms persist for >6 weeks. The presentation of chronic PSII is characterized by chronic pain and implant loosening as well as neurological deficits. In comparison with acute PSII, typical signs of infection such as fever, pus, redness or acute exacerbated pain are regularly missing. Therefore, the diagnosis of chronic PSII is frequently made after the revision spine surgery by the microbiological and histopathological examination of intraoperatively collected peri-implant tissues or removed spinal implants. It has been shown that positive cultures were found in up to 45% of previous expected aseptic revision spine surgeries, even if not every positive culture is equal to the diagnosis of a PSII (21). The diagnosis of PSII is complicated by the fact that chronic PSII is commonly caused by low-virulent pathogens. Some of them are able to build a so-called biofilm surrounding the spinal implant wherein the microorganisms live with a reduced metabolism and are protected against endogenous immune reactions. This may lead to false-negative tissue cultures (22,23). Thus, in the last years the sonication of removed implants has been developed to the gold standard for the evidence of microorganisms in periprosthetic and PSIIs, because it shows an enhanced removal of the biofilm from the implant and makes the microorganisms accessible for microbiological incubation (13,14,24). Its sensitivity in diagnosis of PSII has been reported with more than 90% and consequently much higher than peri-implant tissue cultures or histopathological examination. Commonly found pathogens are coagulase-negative staphylococci (especially S. epidermidis) and Cutibacterium acnes (formerly Propionibacterium acnes) (13,14).
Especially in late-onset PSII caused by low-virulent microorganisms, preoperative laboratory parameter such as C-reactive protein are reported with a high rate of misdiagnosing and insufficient specificity in case of a PSII (25). Blood cultures may indicate haematogenous infection and should be collected possible in case of systemic infection signs such as fever, shivering, sepsis or suspected or confirmed spondylodiscitis before initializing an antimicrobial therapy. Preoperative performed imaging (X-ray, CT scan) may show indirect signs of infection, for example screw loosening or pseudarthrosis. MRI should be performed if purulence or spondylodiscitis are suspected.
Treatment algorithm of PSII
The correct treatment of PSII is essential for an adequate outcome of the patients, prevention of new infections and long-term retention of the spinal implants, because it has been shown that PSII can lead to mechanic failure of the implants like screw loosening or pseudarthrosis and subsequently to new symptoms of the patients and in the end the need of revision spine surgery (26,27). Positive cultures have been found in up to 19% of presumed aseptic pseudarthrosis after lumbar spinal fusion surgery (28). As with the diagnosis of PSII, evidence-based guidelines for the treatment of PSIIs are still not existing and clinical data, especially prospective clinical studies are rare. The treatment of PSII includes surgical and antibiotic strategies with target of eradication or at least suppression of the causative microorganisms.
Surgical treatment strategies for PSII
In the surgical treatment of PSII there are three established options existing: removal of spinal implants, retention of spinal implants with radical debridement and the exchange of the spinal implants. The complete removal of spinal implants in case of PSII is only feasible, if the implants can be removed without the risk of a possible instability spine, e.g., after instrumented spinal fusion in case of traumatic spinal fractures with completely fused spinal segments. After removal, a radical debridement of surrounding tissue should be performed and an antibiotic treatment should be initiated.
The retention of spinal implants in case of PSII is only recommended in early-onset PSII (10). In revision spine surgery with diagnosis of early-onset PSII, the spinal implants should be proved for loosening and if needed single screws should be exchanged. Otherwise, all implants can be retained followed by a radical debridement of the surrounding tissues. However, peri-implant tissue sample should be collected before debridement for microbiological examination for the evidence of causative pathogens and subsequently a targeted antibiotic treatment.
The exchange of the spinal implants is usually done in a single-staged procedure in case of early-onset PSII with multiple loosening of the spinal implants or in case of late-onset PSII without prospect of completely removal for a longer time. After removal, peri-implant tissue cultures should be collected and a radical debridement should be performed. The removed spinal implant should be sent to the sonication for identification of the causative pathogens. After the procedure, first empiric and over time a targeted antibiotic therapy should be initiated. A two-sided exchange of spinal implants in case of PSII is only done in exceptional cases, in which a complete immobilization of the involved segments is possible, e.g., in cervical spine, with an intravenous antibiotic treatment between removal and new implantation surgery. There’s only rare information about the effectiveness of the used implant materials in revision spine surgery. It’s reported that pure titanium cages have lower rate of infection than stainless steel. Polyetheretherketone (PEEK) shows a high propensity for biofilm formation and consequently for infection. Therefore, in revision spine surgery of late-onset PSII, pure titanium cages could reduce the risk of a re-infection compared to PEEK cages (10).
Antibiotic treatment strategies of PSII
The choice of the antibiotic agent(s) in best case should cover all causative pathogens. Because in many cases the exact microorganisms are still not known when the antibiotic treatment is initiated, the antibiotic treatment should begin with an empiric antibiotic regime, covering all expected pathogens. For that, local pathogen spectra, individual risk factors and clinical presentation (e.g., acute or chronic) should bear in mind. Antibiotic treatment always should be initiated after collecting microbiological samples such as blood cultures, peri-implant tissue cultures or removal of spinal implants, because microbiological examination of these samples could be influenced by a previous antibiotic treatment and subsequently their rate of false-negative results could be increased (29). Antibiotic therapy in case of PSII should start with an intravenous application of the antibiotic agents for two weeks, because of the higher bioavailability and a high rate of bacteremia (19,30). Suggestions for empiric antibiotic treatment by the PRO-IMPLANT Foundation are shown in Table 2.
As soon as the causative pathogens are identified by the microbiological examinations, the antibiotic treatment should be switched from empiric to a targeted antibiotic therapy. The targeted treatment should also start with intravenous application of the antibiotic agent(s) for at least 2 weeks. Following this, the treatment can be switched to oral application for an individual duration depending on causative pathogen and surgical treatment strategy.
In patients with late-onset PSII in which a removal of the spinal implants is possible without causing an instability of the spine, antibiotic treatment orientates to the antibiotic treatment in case of regular spondylodiscitis. Despite guidelines for this treatment also do not exist, in clinical practice the antibiotic treatment is continued for about 6 weeks. It has been reported that longer duration of antibiotic treatment does not offer any benefits (30-32).
In patients in which a complete removal of the spinal implants is not practicable because of the risk of an instability of the spine and possible neurological deficits, the antibiotic treatment is more challenging (33,34). Published data regarding the optimized treatment duration in case of PSII are rare und current references are based on studies and experiences in periprosthetic joint infections. After the duration of antibiotic treatment in those cases were between 6 months and 2 years over a long period founded on observational studies, recent studies show good results with an antibiotic treatment for 12 weeks (19,20,33,35). In case of problematic microorganisms an individual long-term suppression is still recommended until removal of spinal implants is possible. Suggestions for targeted antibiotic therapy by the PRO-IMPLANT Foundation can be found in the article “Antibiotic treatment of postoperative spinal implant infections” of this focused issue.
In summary, the treatment of PSII always requires a combination of surgical and antibiotic therapy. In case of stability without further internal fixation, removal and antibiotic therapy similar to the therapy of spondylodiscitis is the treatment of choice. In early-onset PSII, retention of the spinal implants with radical debridement and antibiotic eradication of causative microorganism for 12 weeks in case of unproblematic pathogens is a feasible option of treatment, if the implants are not loosed. Late-onset or chronic PSII requires one-staged exchange of the implants, radical surgical debridement and antibiotic treatment for 12 weeks for unproblematic pathogens. In case of problematic pathogens causing a PSII, duration of the antibiotic treatment is individually and should be initiated after consultation with an infectiologist. A compendious treatment algorithm of PSII is shown in Figures 1 and 2.
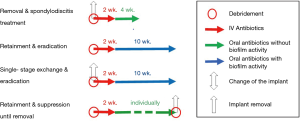
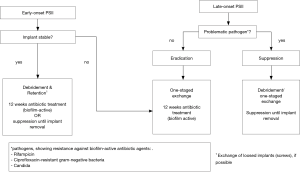
By extension, prophylaxis is an effective tool for therapy of PSII. The North American Spine Society (NASS) recommend preoperative antibiotic prophylaxis using a broad-spectrum antibiotic agent for instrumented spinal surgeries (36). While in uncomplicated simple spine surgery a single-dose antibiotic prophylaxis is recommended, a prolonged postoperative antibiotic treatment in case of complex multi-level spine surgery, e.g., in spinal deformity, is discussable and may reduce the risk of a PSII considering possible negative side effects (37). Furthermore, the local wound application of vancomycin powder is reported with a significant reduced risk for PSII (38,39).
It has been shown that minimally invasive spine surgery (MISS) reduces the risk of surgical site infections (SSIs) in spinal surgery (40,41). Even if this is not investigated especially for low-grade infections without clinical presentation yet, this kind of PSII may be reduced by performing MISS, too.
Conclusions
PSIIs are commonly found after instrumented spinal fusion. Its treatment is challenging the physicians and for optimized results surgical intervention followed by an antibiotic treatment for at least 12 weeks in cases of retained spinal implants is required. In early-onset PSII retention of spinal implants is a feasible option whereas late-onset or chronic PSII requires exchange of the implants. Problematic pathogens with resistance against biofilm-active antibiotic agents can necessitate application of antibiotic agents for months or years with their associated complications. Long-term experiences and data with current treatment concepts are still rare and it needs further investigations to prove their efficiency.
Acknowledgments
This work was supported by the PRO-IMPLANT Foundation, Berlin, Germany (www.pro-implant.org) providing the copyright for their management guidelines in “Pocket Guide”.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Matthias Pumberger) for the series “Postoperative Spinal Implant Infection” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-497). The series “Postoperative Spinal Implant Infection” was commissioned by the editorial office without any funding or sponsorship. MP served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Journal of Spine Surgery from Nov 2018 to Nov 2020. MP also reports personal fees from Aesculap, Medtronic and SpineArt outside the submitted work. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roy-Camille R, Saillant G, Mazel C. Internal fixation of the lumbar spine with pedicle screw plating. Clin Orthop Relat Res 1986.7-17. [Crossref] [PubMed]
- Pumberger M, Chiu YL, Ma Y, et al. National in-hospital morbidity and mortality trends after lumbar fusion surgery between 1998 and 2008. J Bone Joint Surg Br 2012;94:359-64. [Crossref] [PubMed]
- Sivasubramaniam V, Patel HC, Ozdemir BA, et al. Trends in hospital admissions and surgical procedures for degenerative lumbar spine disease in England, A 15-year time-series study. BMJ Open 2015;5:e009011. [Crossref] [PubMed]
- Fingar KR, Stocks C, Weiss AJ, et al. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs, Most Frequent Operating Room Procedures Performed in U.S. Hospitals, 2003-2012: Statistical Brief #186. Agency for Healthcare Research and Quality (US). Rockville (MD), 2006.
- Boody BS, Jenkins TJ, Hashmi SZ, et al. Surgical Site Infections in Spinal Surgery. J Spinal Disord Tech 2015;28:352-62. [Crossref] [PubMed]
- Patel H, Khoury H, Girgenti D, et al. Burden of Surgical Site Infections Associated with Select Spine Operations and Involvement of Staphylococcus aureus. Surg Infect (Larchmt) 2017;18:461-73. [Crossref] [PubMed]
- Pull ter Gunne AF, Mohamed AS, Skolasky RL, et al. The presentation, incidence, etiology, and treatment of surgical site infections after spinal surgery. Spine (Phila Pa 1976) 2010;35:1323-8. [Crossref] [PubMed]
- Saeedinia S, Nouri M, Azarhomayoun A, et al. The incidence and risk factors for surgical site infection after clean spinal operations: A prospective cohort study and review of the literature. Surg Neurol Int 2015;6:154. [Crossref] [PubMed]
- Kurtz SM, Lau E, Ong KL, et al. Infection risk for primary and revision instrumented lumbar spine fusion in the Medicare population. J Neurosurg Spine 2012;17:342-7. [Crossref] [PubMed]
- Kasliwal MK, Tan LA, Traynelis VC. Infection with spinal instrumentation, Review of pathogenesis, diagnosis, prevention, and management. Surg Neurol Int 2013;4:S392-403. [Crossref] [PubMed]
- Pereira BJA, Holanda CVM, de Ribeiro CAA, et al. Spinal surgery for degenerative lumbar spine disease, Predictors of outcome. Clin Neurol Neurosurg 2016;140:1-5. [Crossref] [PubMed]
- Abdul-Jabbar A, Takemoto S, Weber MH, et al. Surgical site infection in spinal surgery, Description of surgical and patient-based risk factors for postoperative infection using administrative claims data. Spine (Phila Pa 1976) 2012;37:1340-5. [Crossref] [PubMed]
- Sampedro MF, Huddleston PM, Piper KE, et al. A biofilm approach to detect bacteria on removed spinal implants. Spine (Phila Pa 1976) 2010;35:1218-24. [Crossref] [PubMed]
- Bürger J, Akgün D, Strube P, et al. Sonication of removed implants improves microbiological diagnosis of postoperative spinal infections. Eur Spine J 2019;28:768-74. [Crossref] [PubMed]
- Baranowska A, Baranowska J, Baranowski P.. Analysis of Reasons for Failure of Surgery for Degenerative Disease of Lumbar Spine. Ortop Traumatol Rehabil 2016;18:117-29. [Crossref] [PubMed]
- Tsai TT, Lee SH, Niu CC, et al. Unplanned revision spinal surgery within a week, A retrospective analysis of surgical causes. BMC Musculoskelet Disord 2016;17:28. [Crossref] [PubMed]
- Yadla S, Ghobrial GM, Campbell PG, et al. Identification of complications that have a significant effect on length of stay after spine surgery and predictive value of 90-day readmission rate. J Neurosurg Spine 2015;23:807-11. [Crossref] [PubMed]
- McCormack RA, Hunter T, Ramos N, et al. An analysis of causes of readmission after spine surgery. Spine (Phila Pa 1976) 2012;37:1260-6. [Crossref] [PubMed]
- Dubée V, Lenoir T, Leflon-Guibout V, et al. Three-month antibiotic therapy for early-onset postoperative spinal implant infections. Clin Infect Dis 2012;55:1481-7. [Crossref] [PubMed]
- Kowalski TJ, Berbari EF, Huddleston PM, et al. The management and outcome of spinal implant infections, Contemporary retrospective cohort study. Clin Infect Dis 2007;44:913-20. [Crossref] [PubMed]
- Pumberger M, Bürger J, Strube P, et al. Unexpected positive cultures in presumed aseptic revision spine surgery using sonication. Bone Joint J 2019;101-B:621-4. [Crossref] [PubMed]
- Karczewski D, Winkler T, Renz N, et al. A standardized interdisciplinary algorithm for the treatment of prosthetic joint infections. Bone Joint J 2019;101-B:132-9. [Crossref] [PubMed]
- Marculescu CE, Berbari EF, Hanssen AD, et al. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin Infect Dis 2006;42:471-8. [Crossref] [PubMed]
- Janz V, Wassilew GI, Hasart O, et al. Evaluation of sonicate fluid cultures in comparison to histological analysis of the periprosthetic membrane for the detection of periprosthetic joint infection. Int Orthop 2013;37:931-6. [Crossref] [PubMed]
- Akgün D, Bürger J, Pumberger M, et al. C-reactive protein misdiagnoses delayed postoperative spinal implant infections in patients with low-virulent microorganisms. Eur Spine J 2019;28:2990-5. [Crossref] [PubMed]
- Leitner L, Malaj I, Sadoghi P, et al. Pedicle screw loosening is correlated to chronic subclinical deep implant infection, A retrospective database analysis. Eur Spine J 2018;27:2529-35. [Crossref] [PubMed]
- Prinz V, Bayerl S, Renz N, et al. High frequency of low-virulent microorganisms detected by sonication of pedicle screws, A potential cause for implant failure. J Neurosurg Spine 2019;31:424-9. [Crossref] [PubMed]
- Shifflett GD, Bjerke-Kroll BT, Nwachukwu BU, et al. Microbiologic profile of infections in presumed aseptic revision spine surgery. Eur Spine J 2016;25:3902-7. [Crossref] [PubMed]
- Dowdell J, Brochin R, Kim J, et al. Postoperative Spine Infection, Diagnosis and Management. Global Spine J 2018;8:37S-43S. [Crossref] [PubMed]
- Sobottke R, Seifert H, Fätkenheuer G, et al. Current diagnosis and treatment of spondylodiscitis. Dtsch Arztebl Int 2008;105:181-7. [PubMed]
- Rutges JPHJ, Kempen DH, van Dijk M, et al. Outcome of conservative and surgical treatment of pyogenic spondylodiscitis, A systematic literature review. Eur Spine J 2016;25:983-99. [Crossref] [PubMed]
- Jaramillo-de la Torre JJ, Bohinski RJ, et al. Vertebral osteomyelitis. Neurosurg Clin N Am 2006;17:339-51. vii.. [Crossref] [PubMed]
- Sierra-Hoffman M, Jinadatha C, Carpenter JL, et al. Postoperative instrumented spine infections, A retrospective review. South Med J 2010;103:25-30. [Crossref] [PubMed]
- Quiñones-Hinojosa A, Jun P, Jacobs R, et al. General principles in the medical and surgical management of spinal infections, A multidisciplinary approach. Neurosurg Focus 2004;17:E1. [PubMed]
- Collins I, Wilson-MacDonald J, Chami G, et al. The diagnosis and management of infection following instrumented spinal fusion. Eur Spine J 2008;17:445-50. [Crossref] [PubMed]
- Shaffer WO, Baisden JL, Fernand R, et al. An evidence-based clinical guideline for antibiotic prophylaxis in spine surgery. Spine J 2013;13:1387-92. [Crossref] [PubMed]
- Ho AL, Stienen MN, Ratliff JK. Letter, Antibiotic Stewardship and Single-Dose Antibiotic Prophylaxis: A Word of Caution. Neurosurgery 2020;86:E360-E361. [Crossref] [PubMed]
- Gaviola ML, McMillian WD, Ames SE, et al. A Retrospective Study on the Protective Effects of Topical Vancomycin in Patients Undergoing Multilevel Spinal Fusion. Pharmacotherapy 2016;36:19-25. [Crossref] [PubMed]
- Haimoto S, Schär RT, Nishimura Y, et al. Reduction in surgical site infection with suprafascial intrawound application of vancomycin powder in instrumented posterior spinal fusion: a retrospective case-control study. J Neurosurg Spine 2018;29:193-8. [Crossref] [PubMed]
- O'Toole JE, Eichholz KM, Fessler RG. Surgical site infection rates after minimally invasive spinal surgery. J Neurosurg Spine 2009;11:471-6. [Crossref] [PubMed]
- Kulkarni AG, Patel RS, Dutta S. Does Minimally Invasive Spine Surgery Minimize Surgical Site Infections? Asian Spine J 2016;10:1000-6. [Crossref] [PubMed]



