
Antibiotic treatment of postoperative spinal implant infections
Introduction
Orthopaedic implants are used in a variety of bone injuries and joint illnesses. In the spine, internal fixation devices have become a mainstay in the treatment of acute injuries such as fractures, as well as chronic degenerative changes such as osteochondrosis, or congenital deformities such as scoliosis. However, the incorporation of foreign materials into the human body is accompanied by the risk of infection. Implant-associated infections are among the most fearsome complications in the field of orthopaedic surgery. Despite ongoing advances regarding operation techniques and sterility levels, infection rates after spinal instrumentation are still reported up to 20% and are the most common reason for unplanned revision spine surgery within 30 days after index surgery (1-3). In revision spine surgery with removal of spinal implants postoperative spinal implant infection (PSII) has been reported to be found in up to 27% (4,5). One reason is related to the ability of bacteria to attach and form a biofilm on the surface of implants. The formation of such a biofilm dramatically reduces the bacteria’s susceptibility to natural immune defence mechanisms as well as antibiotics. In addition, it also hinders the detection of bacteria, thereby creating a considerable medical challenge regarding both diagnostics and treatment. As a result, implant-associated infections may cause severe morbidity for affected patients as well as considerable costs for the health care system.
Regarding the treatment of PSII, most cases require surgical revision (see article “Surgical Revision Strategies” in this issue). However, surgical interventions always have to be accompanied by an adequate antibiotic treatment protocol in order to achieve efficient pathogen eradication and good clinical results. Vertebral bodies are composed of highly vascularized bone, enabling easy penetration and diffusion to the infected site (6,7). In clinical practice choice of an adequate treatment strategy is challenged by the lack of published guidelines on the treatment of PSII and paucity of respective literature.
In this review, we aim to summarize the existing literature on antibiotic treatment of PSII and to provide guidance for treating doctors to make sensible decisions about antibiotic treatments for improved clinical outcomes.
Classification and pathogens
Implant-associated infections can be divided into different categories according to time of symptom onset (acute or chronic) and origin of the pathogens (postinterventional, haematogenous, per continuitatem). These categories are typically characterized by distinct clinical presentations and causative microorganisms, and require specific treatment protocols. Major characteristics of acute and chronic infections are summarized in Table 1.

Full table
Acute implant-associated infections, occurring within 6 weeks after surgery or with a symptom onset <6 weeks ago, are usually caused by highly virulent pathogens. Patients with acute infections often present with acute pain, fever, prolonged wound drainage (>7–10 days) or acute neurologic deficits. The majority of early-onset infections has been reported to be caused by Staphylococcus aureus, followed by streptococci, enterococci, and Gram-negative bacilli (6,8). Treatment of early-onset infections is complicated by the fact that the operated spinal segments have not yet had enough time for fusion to take place. Therefore, the implant is often still necessary to maintain stability of the spine and cannot be removed, which also influences antibiotic treatment choices.
In contrast, chronic infections typically cause symptoms such as chronic pain and implant loosening, but may as well lead to neurologic deficits. Typical clinical signs of infection such as fever or pus are usually missing. In many cases, the diagnosis is made only based on the microbiological and histopathological examination of intraoperatively collected tissue samples and removed implants. Chronic infections are mostly caused by low-virulent pathogens or can be culture-negative. In the past, there has been some debate whether microbial agents are the cause of late-onset spinal implant infection or if late-onset drainage is rather a result of aseptic inflammation from metal corrosion, with cultures positive for low-virulent organisms being of no pathogenic significance (8,9). This thesis was supported by some studies reporting >80% of late-onset “infections” to be culture negative (10). In contrast, other authors have described pathogen detection rates of >90% using extended culture incubation times (11,12). Thus, the formerly reported high number of culture negative samples may be due to insufficient sensitivity or incubation times. It has also been suggested that the environment created by postoperative sterile inflammatory processes may be favourable for the growth of low-virulence organisms (1,13). Typical pathogens of chronic infections include coagulase-negative staphylococci and Cutibacterium (formerly Propionibacterium) spp. Late-onset infections are primarily caused by organisms that are able to produce biofilm on the implant. The presence of biofilm hinders pathogen detection and can make eradication difficult without implant removal, similar to other bone and joint infections involving prosthesis (8,14,15). In many patients with late-onset infections the operated segments have already fused allowing for removal of the implants.
Pathogenesis
One of the main reasons for the highly increased risk of infection after spinal instrumentation lies in the formation of biofilms. A biofilm is a structured aggregation of bacteria encased in a self-produced matrix of extracellular polysaccharides that adheres to a surface (16). The surface of materials commonly used for spinal implants such as titanium, stainless steel, various polymeric biomaterials, and polymethylmethacrylate (PMMA) cement are all susceptible to colonization by biofilm-forming bacteria. There are three different ways in which an implant can get colonized: (I) within the perioperative period, e.g., via intraoperative inoculation; (II) haematogenously by pathogens from other infected foci, e.g., respiratory or urinary tract infection; and (III) per continuitatem, e.g., due to infected surrounding soft tissues. The development of a biofilm on orthopaedic implants can be divided into the four stages cell adhesion, cell aggregation, biofilm maturation, and cellular detachment (17). Once a biofilm has formed, bacteria display a highly increased resistance against both endogenous immune defense and antibiotics. Even though the responsible mechanisms are not yet fully understood, the existence of slow or non-growing cells within the biofilm is thought to play an important role (18).
Antibiotic treatment
An adequate management of PSII always involves a surgical intervention together with antibiotic treatment. For eradication of an implant-associated infection antibiotic treatment should be active against all causative pathogens in their biofilm form (19). In many cases, the exact pathogen(s) is/are unknown at the time of surgery. Therefore, empiric antibiotic regime is given initially that covers the most common and expected pathogens. Empiric treatment of PSII should cover staphylococci, streptococci, enterococci and Gram-negative bacilli. Polymicrobial infections are common in both, early-onset and late-onset PSII with rates of up to 50% (6,8,20,21). Antimicrobial resistant pathogens are rather uncommon, although their incidence is increasing (6,22,23). Antibiotic therapy should only be initiated after tissue samples for microbiological culture have been obtained (24).
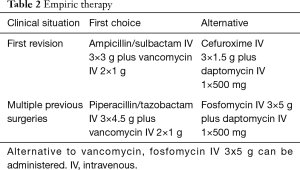
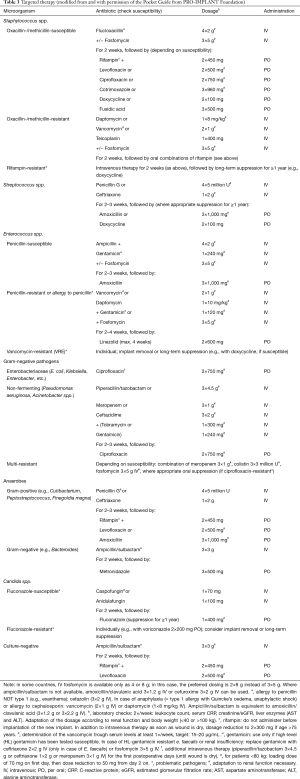
Suggestions for empiric and targeted therapy according to the PRO-IMPLANT Foundation are presented in Tables 2,3. Antibiotic therapy is initiated by intravenous (IV) administration for the first 1–2 weeks to achieve sufficient tissue concentration in short time (6,25). When there is a clinical (no wound secretion) and laboratory response, which is usually after 1–2 weeks, treatment can be oral administration. The duration of the oral antibiotic treatment should be adapted depending on the causative pathogen and the clinical presentation of the patient.
Full table
Full table
In cases of late-onset PSII in which the implant can be removed without compromising the stability of the spine, treatment resembles therapeutic approaches of regular spondylodiscitis. Although there are no published guidelines, general consensus is to continue antibiotic treatment for around 6 weeks. Longer treatment durations may not bring additional benefit as similar outcomes have been reported (25-27). If the implants can be removed, oral biofilm-active antibiotics should be avoided in order to avoid resistancies.
In patients where implant removal is not feasible, antibiotic treatment is more demanding, especially because data on the optimal treatment duration is scarce (28,29). In the past, patients with PSII were usually administered antibiotic therapy for very long durations of more than 6 months, and in some cases up to 2 years on the basis of a few observational studies (6,8,29). However, current studies suggest good results for shorter antibiotic treatment with a total duration of 12 weeks (6,12). In some patients, particularly in those with problematic pathogens (i.e., pathogens resistant to biofilm-active antibiotics, e.g., rifampin-resistant staphylococci, ciprofloxacin-resistant Gram-negative bacteria and fungal infection), a long-term suppression therapy may be advisable until implant removal is possible. In general, if the implants are retained or exchanged an oral biofilm-active antibiotic treatment should only be started if an eradication is intended and not before the wound is dry. Figure 1 depicts the different treatment strategies.
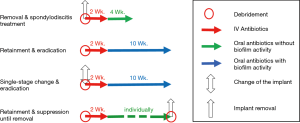
In cases of uncertainty it is recommended to consult an infectious disease doctor for co-management of the patient and to guide antibiotic therapy (24).
Conclusions
Adequate therapy of PSII is highly complex and challenging for physicians and patients alike. To reduce morbidity and mortality, continuous careful evaluation of treatment strategies is of uttermost importance. Data regarding optimal management are still scarce, but recent studies have shown good results for a total treatment duration of 12 weeks with IV followed by oral antibiotics for most patients. In patients with problematic pathogens, treatment duration has to be prolonged individually, possibly until removal of the implant seems feasible.
Acknowledgments
Dr. Palmowski is participant in the BIH-Charité Junior Clinician Scientist Program funded by the Charité-Universitätsmedizin Berlin and the Berlin Institute of Health. The antibiotic recommendations are modified from the Pocket Guide of the PRO-IMPLANT Foundation, Berlin, Germany (www.pro-implant-foundation.org).
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Matthias Pumberger) for the series “Postoperative Spinal Implant Infection” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss-20-456). The series “Postoperative Spinal Implant Infection” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kasliwal MK, Tan LA, Traynelis VC. Infection with spinal instrumentation: Review of pathogenesis, diagnosis, prevention, and management. Surg Neurol Int 2013;4:S392-403. [Crossref] [PubMed]
- Parchi PD, Evangelisti G, Andreani L, et al. Postoperative Spine Infections. Orthop Rev (Pavia) 2015;7:5900. [Crossref] [PubMed]
- Baranowska A, Baranowska J, Baranowski P.. Analysis of Reasons for Failure of Surgery for Degenerative Disease of Lumbar Spine. Ortop Traumatol Rehabil 2016;18:117-29. [Crossref] [PubMed]
- Burger J, Akgun D, Strube P, et al. Sonication of removed implants improves microbiological diagnosis of postoperative spinal infections. Eur Spine J 2019;28:768-74. [Crossref] [PubMed]
- Sampedro MF, Huddleston PM, Piper KE, et al. A biofilm approach to detect bacteria on removed spinal implants. Spine (Phila Pa 1976) 2010;35:1218-24. [Crossref] [PubMed]
- Dubee V, Lenoir T, Leflon-Guibout V, et al. Three-month antibiotic therapy for early-onset postoperative spinal implant infections. Clin Infect Dis 2012;55:1481-7. [Crossref] [PubMed]
- Wiley AM, Trueta J. The vascular anatomy of the spine and its relationship to pyogenic vertebral osteomyelitis. J Bone Joint Surg Br 1959;41-B:796-809. [Crossref] [PubMed]
- Kowalski TJ, Berbari EF, Huddleston PM, et al. The management and outcome of spinal implant infections: contemporary retrospective cohort study. Clin Infect Dis 2007;44:913-20. [Crossref] [PubMed]
- Dietz FR. Point of View. Spine 2001;26:1995-6. [Crossref]
- Muschik M, Luck W, Schlenzka D. Implant removal for late-developing infection after instrumented posterior spinal fusion for scoliosis: reinstrumentation reduces loss of correction. A retrospective analysis of 45 cases. Eur Spine J 2004;13:645-51. [Crossref] [PubMed]
- Clark CE, Shufflebarger HL. Late-developing infection in instrumented idiopathic scoliosis. Spine (Phila Pa 1976) 1999;24:1909-12. [Crossref] [PubMed]
- Collins I, Wilson-MacDonald J, Chami G, et al. The diagnosis and management of infection following instrumented spinal fusion. Eur Spine J 2008;17:445-50. [Crossref] [PubMed]
- Haidar R, Najjar M, Der Boghossian A, et al. Propionibacterium acnes causing delayed postoperative spine infection Scand J Infect Dis 2010;42:405-11. review. [Crossref] [PubMed]
- Marculescu CE, Berbari EF, Hanssen AD, et al. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin Infect Dis 2006;42:471-8. [Crossref] [PubMed]
- Karczewski D, Winkler T, Renz N, et al. A standardized interdisciplinary algorithm for the treatment of prosthetic joint infections. Bone Joint J 2019;101-B:132-9. [Crossref] [PubMed]
- Donlan RM. Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis 2001;33:1387-92. [Crossref] [PubMed]
- Gbejuade HO, Lovering AM, Webb JC. The role of microbial biofilms in prosthetic joint infections. Acta Orthop 2015;86:147-58. [Crossref] [PubMed]
- Li C, Renz N, Trampuz A.. Management of Periprosthetic Joint Infection. Hip Pelvis 2018;30:138-46. [Crossref] [PubMed]
- Köder K, Hardt S, Gellert MS, et al. Outcome of spinal implant-associated infections treated with or without biofilm-active antibiotics: results from a 10-year cohort study. Infection 2020;48:559-68. [Crossref] [PubMed]
- Levi AD, Dickman CA, Sonntag VK. Management of postoperative infections after spinal instrumentation. J Neurosurg 1997;86:975-80. [Crossref] [PubMed]
- Rohmiller MT, Akbarnia BA, Raiszadeh K, et al. Closed suction irrigation for the treatment of postoperative wound infections following posterior spinal fusion and instrumentation. Spine (Phila Pa 1976) 2010;35:642-6. [Crossref] [PubMed]
- Kim JI, Suh KT, Kim SJ, et al. Implant removal for the management of infection after instrumented spinal fusion. J Spinal Disord Tech 2010;23:258-65. [Crossref] [PubMed]
- Klekamp J, Spengler DM, McNamara MJ, et al. Risk factors associated with methicillin-resistant staphylococcal wound infection after spinal surgery. J Spinal Disord 1999;12:187-91. [PubMed]
- Dowdell J, Brochin R, Kim J, et al. Postoperative Spine Infection: Diagnosis and Management. Global Spine J 2018;8:37S-43S. [Crossref] [PubMed]
- Sobottke R, Seifert H, Fatkenheuer G, et al. Current diagnosis and treatment of spondylodiscitis. Dtsch Arztebl Int 2008;105:181-7. [PubMed]
- Jaramillo-de la Torre JJ, Bohinski RJ, Kuntz C 4th. Vertebral osteomyelitis. Neurosurg Clin N Am 2006;17:339-51. vii.. [Crossref] [PubMed]
- Rutges JP, Kempen DH, van Dijk M, et al. Outcome of conservative and surgical treatment of pyogenic spondylodiscitis: a systematic literature review. Eur Spine J 2016;25:983-99. [Crossref] [PubMed]
- Quinones-Hinojosa A, Jun P, Jacobs R, et al. General principles in the medical and surgical management of spinal infections: a multidisciplinary approach. Neurosurg Focus 2004;17:E1. [PubMed]
- Sierra-Hoffman M, Jinadatha C, Carpenter JL, et al. Postoperative instrumented spine infections: a retrospective review. South Med J 2010;103:25-30. [Crossref] [PubMed]


