Is dental prophylaxis required following spinal fusion?—a systematic review and call for evidence
Introduction
The Agency for Healthcare Research and Quality reported in 2011 that approximately 488,000 spinal fusions are performed each year in the United States (1). This represented an increase of 70% over a 10-year period starting in 2001 (2). With an aging population increasingly active well into their sixties, seventies, and eighties, the number of spinal fusions performed each year is expected to continue to rise. The average age of patients undergoing fusion is 54.2 years, so in addition to a growing patient population, lengthening life expectancies will mean that these patients’ other care providers will need to become familiar with the unique challenges of providing care over the remaining decades of life after a spinal fusion (3).
A point of particular controversy has remained the question of antimicrobial prophylaxis for patients with a history of spinal instrumentation undergoing invasive procedures on an outpatient basis. These procedures, such as routine dental work, carry a risk of introducing transient bacteremia. Although it has never been definitively proven in human models, animal models demonstrate that it remains a plausible theory that bacteremia introduced from the oral cavity may result in hematogenous late onset infection of the instrumentation (4,5).
Although infection of spinal instrumentation appears to be rare, such complications carry a significant burden and risk (6). Infected implants have the potential to become encased in a biofilm and difficult to treat (7). An active infection may cause systemic symptoms and will occasionally require additional surgery to debride or even remove the infected implant. These complications present inherent risk to health and financial burden to both patients and the healthcare system as they often require one or more reoperations and long-term antibiotics (8).
It is standard practice to utilize perioperative antibiotics to protect against infections acquired during spinal fusion surgery itself, yet there remains no consensus on their prophylactic use in patients with a history of prosthetic installations undergoing maintenance healthcare procedures with a risk of introducing bacteremia. Patients with a history of instrumented spinal fusions who ask their healthcare or dental providers for guidance are likely to receive contradictory recommendations. For this reason, it is important to become aware of the evidence associated with the risk of hematogenous infection of spinal instrumentation and the efficacy of antimicrobial prophylaxis to protect patients from this complication when undergoing routine dental procedures. We therefore sought to perform a systematic review to evaluate the available evidence assessing patients with spinal fusions undergoing dental procedures with or without prophylactic antibiotics.
Methods
We performed a systematic review according to guidelines set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). We searched PubMed, Web of Science, Cochrane Library, and EMBASE databases from inception to March 2019 using the key words [Spondylodesis OR Spinal Fusion OR Spine OR Spine Surgery OR Spinal Surgery OR Decompression OR Laminectomy OR Lumbar OR Thoracic OR Cervical] AND [Dental OR Dentist] AND [Infection OR Antibiotic OR Prophylaxis OR Bacteremia OR Antimicrobial prophylaxis OR Abscess] in all possible combinations. No filters were applied to limit retrieval by study type or date of publication.
Eligible studies included patients with a history of spinal surgery treated with antimicrobial prophylaxis in preparation for dental procedures. Two reviewers independently assessed the eligibility of potential studies and extracted data. Outcomes of interest were the indications and efficacy of antimicrobial prophylaxis to protect against infection of spinal prostheses with dental origin.
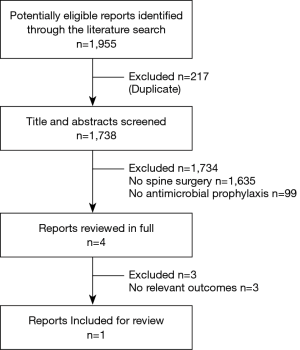
Search results are shown in Figure 1. Initial search resulted in 1,955 studies, which were screened by two independent reviewers. In the first level of screening, titles and abstracts were assessed for potentially relevant articles which were retrieved for potential inclusion. A total of 1,951 studies were removed as they were either not applicable or duplicate, resulting in an inclusion of four articles for secondary review. A full text review was conducted to ensure retrieved articles were appropriate for inclusion. After this level of review, only a single article was deemed appropriate. The other two articles were excluded as they did not address either efficacy or indications for the use of antimicrobial prophylaxis against infections of dental origin. One reviewer then assessed each study to determine strength of guidelines and the results were synthesized through narrative description.
Results
After a thorough systematic review of the literature, it was determined that no objective studies were found on this topic. Existing literature is limited to a single expert survey published in the Journal of Clinical Neuroscience in 2016 (9). This was an expert survey (Level IV evidence) which revealed that approximately two-thirds of spine surgeons would not recommend antimicrobial prophylaxis for patients with a history of uncomplicated lumbar fusion (Figure 2). In patients with history of revisions surgeries, obesity, smoking, diabetes rates increased to 30–40% recommending antibiotics (9).

A similar survey found that approximately seventy percent of dental providers would recommend antimicrobial prophylaxis to patients for at least 2 years after a similar procedure (10). These surveys, as well as objective studies and professional organization guidelines on dental prophylaxis in patients with total hip and knee replacements, were reviewed to add context to the controversy.
Discussion
There is a significant paucity of literature regarding dental prophylaxis in spine surgery patients. This systematic review revealed no objective studies of the efficacy, indication, or utility for antimicrobial prophylaxis to prevent infection of spinal instrumentation caused by hematogenous seeding of dental origin. Despite reported cases of spinal infections from presumed dental origin, the preponderance of evidence for hematogenous seeding of orthopedic prosthesis remains a 1981 study conducted on rabbits in which a large dose of Staphylococcus Aureus was injected into the test animals and demonstrated the potential for hematogenous seeding onto the prosthesis (4,11).
Given the lack of clear, evidence-based guidelines, opinions on the best practices for treatment of patients with a history of spinal instrumentation undergoing dental procedures with the risk of bacteremia remains divided. Standardized surveys reveal that, despite some differences in provider recommendations in certain situations, approximately two-thirds of surgeons specializing in the spine would not prescribe prophylactic antibiotics for patients with a history of uncomplicated lumbar fusion (9). Although this represents a majority opinion, it still leaves a significant population of physicians in disagreement. Dental providers are similarly split. However, in contrast to their physician colleagues, the majority of surveyed dentists recommended prophylaxis with as many as 72% supporting antimicrobial prophylaxis for at least the first 2 years after a prosthetic joint was installed (10).
Disagreement may arise, in part, due to the shifting recommendations of the American Academy of Orthopaedic Surgeons (AAOS). Beginning in 1997, the AAOS, in conjunction with the American Dental Association (ADA), released joint clinical practice guidelines on the prevention of orthopaedic implant infection in patients undergoing dental procedures (12). As recently as 2003 the guidelines concluded “the risk/benefit and cost/effectiveness ratios fail to justify the administration of routine antibiotic prophylaxis” before seemingly changing their position in 2009 to recommend “Given the potential adverse outcomes and cost of treating an infected joint replacement, the AAOS recommends that clinicians consider antimicrobial prophylaxis for all total joint patients prior to any procedure that may cause bacteremia.” (13). The newest edition of the guidelines published in 2016 addressed the problem with a more granular approach stipulating 64 different clinical scenarios including variables such as immunocompromised status, history of prosthetic joint infection, and time since joint installation to determine when dental prophylaxis might be indicated (14). Limitations of this guideline remain that it addresses total knee and hip replacements rather than spinal fusions, and it relies on surveyed expert opinion rather than objective evidence-based practices.
Without extensive, objective evidence to support the theory that dental procedures cause infection of orthopedic prosthetics, dental prophylaxis is often justified by the frequency, cost, and devastating impact that infections can have on patients (15). Despite these concerns, literature addressing the risk of hematogenous infection of orthopaedic implants resulting invasive outpatient procedures indicate a movement away from the prescription of antimicrobial prophylaxis. Although these studies overwhelmingly focus on total hip and knee replacements, certain arguments made by the authors might be generalized to similar cases in the spine. Many of the arguments against routine prophylaxis fall into a few categories:
The risks of prescribing antimicrobial prophylaxis outweigh the benefit
From an epidemiologic view, many thousands of patients would likely have to undergo prophylaxis to prevent a single infection. With so many patients receiving treatment, the risk of adverse events (e.g., drug interactions, allergic reactions, and bacterial resistance) would likely dictate the risk-benefit analysis (16).
Antimicrobial prophylaxis is not a cost-effective prevention of orthopaedic joint infections
Although the cost of antibiotics is low for each individual, routine prescribing of antimicrobial prophylaxis would be projected to cost the American healthcare system significantly more than the burden incurred by infected prosthesis (17,18).
Dental prophylaxis is unnecessary
Although dental work carries the risk of introducing transient bacteremia, so do normal daily activities including mastication and teeth cleaning. In fact, the cumulative exposure introduced by normal daily activities is almost certainly higher than the risk associated with invasive dental procedures (19). Rather than concerning ourselves with the one-time exposure of dental care, we may be better served by encouraging patients to maintain good oral hygiene.
Conclusions
The objective of this review was to synthesize a comprehensive summary of the published literature in order to provide recommendations on the use of antimicrobial prophylaxis before invasive dental procedures in patients with a history of spinal fusion. The low level of evidence produced by this systematic review renders it impossible to determine an evidence-based recommendation.
Likely due to the lack of literature addressing this subject, expert opinion remains divided and healthcare providers in different fields are still likely to give patients conflicting advice. Although there has been a recent movement away from recommending antimicrobial prophylaxis before dental work in patients with other forms of orthopaedic prosthesis, the gap in the literature addressing spine patients represents an important question that requires more targeted and specific research to satisfactorily answer. Future research should attempt to establish the frequency and burden of spinal instrumentation infections with dental origin, the costs, risks, and efficacy of antimicrobial prophylaxis in protecting against these events, and the relative risk of invasive dental procedures compared to normal daily activities in the introduction of hematogenous spinal infections.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Weiss AJ, Elixhauser A, Andrews RM. Characteristics of Operating Room Procedures in U.S. Hospitals, 2011. HCUP Statistical Brief #170. Rockville, MD: Agency for Healthcare Research and Quality, 2014.
- Weiss AJ, Elixhauser A. Trends in Operating Room Procedures in U.S. Hospitals, 2001—2011. HCUP Statistical Brief #171. Rockville, MD: Agency for Healthcare Research and Quality, 2014.
- Rajaee SS, Bae HW, Kanim LE, et al. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976) 2012;37:67-76. [Crossref] [PubMed]
- Blomgren G. Hematogenous infection of total joint replacement. An experimental study in the rabbit. Acta Orthop Scand Suppl 1981;187:1-64. [Crossref] [PubMed]
- Southwood RT, Rice JL, McDonald PJ, et al. Infection in experimental arthroplasties. Clin Orthop Relat Res 1987.33-6. [PubMed]
- Chaichana KL, Bydon M, Santiago-Dieppa DR, et al. Risk of infection following posterior instrumented lumbar fusion for degenerative spine disease in 817 consecutive cases. J Neurosurg Spine 2014;20:45-52. [Crossref] [PubMed]
- Song Z, Borgwardt L, Høiby N, et al. Prosthesis infections after orthopedic joint replacement: the possible role of bacterial biofilms. Orthop Rev (Pavia) 2013;5:65-71. [Crossref] [PubMed]
- Hernández-Vaquero D, Fernández-Fairen M, Torres A, et al. Treatment of periprosthetic infections: an economic analysis. ScientificWorldJournal 2013;2013:821650.
- Lewkonia P, DiPaola C, Street J. Incidence and risk of delayed surgical site infection following instrumented lumbar spine fusion. J Clin Neurosci 2016;23:76-80. [Crossref] [PubMed]
- Spittle LS, Muzzin KB, Campbell PR, et al. Current prescribing Practices for Antibiotic Prophylaxis: A Survey of Dental Practitioners. J Contemp Dent Pract 2017;18:559-66. [Crossref] [PubMed]
- Kaye ID, Protopsaltis TS. Cervical Facet Joint Infection and Associated Epidural Abscess with Streptococcus intermedius from a Dental Infection Origin A Case Report and Review. Bull Hosp Jt Dis (2013) 2016;74:237-43. [PubMed]
- Advisory statement. Antibiotic prophylaxis for dental patients with total joint replacements. American Dental Association; American Academy of Orthopaedic Surgeons. J Am Dent Assoc 1997;128:1004-8. [Crossref] [PubMed]
- Jevsevar DS, Abt E. The new AAOS-ADA clinical practice guideline on Prevention of Orthopaedic Implant Infection in Patients Undergoing Dental Procedures. J Am Acad Orthop Surg 2013;21:195-7. [PubMed]
- Quinn RH, Murray JN, Pezold R, et al. The American Academy of Orthopaedic Surgeons Appropriate Use Criteria for the Management of Patients with Orthopaedic Implants Undergoing Dental Procedures. J Bone Joint Surg Am 2017;99:161-3. [Crossref] [PubMed]
- Gillespie WJ. Infection in Total Joint Replacement. Infectious Disease Clinics of North America. U.S. National Library of Medicine, Sept 1990.
- Sendi P, Uçkay I, Suvà D, et al. Antibiotic Prophylaxis During Dental Procedures in Patients with Prosthetic Joints. J Bone Jt Infect 2016;1:42-9. [Crossref] [PubMed]
- Lockhart PB, Blizzard J, Maslow AL, et al. Drug cost implications for antibiotic prophylaxis for dental procedures. Oral Surg Oral Med Oral Pathol Oral Radiol 2013;115:345-53. [Crossref] [PubMed]
- Skaar DD, Park T, Swiontkowski MF, et al. Cost-effectiveness of antibiotic prophylaxis for dental patients with prosthetic joints: Comparisons of antibiotic regimens for patients with total hip arthroplasty. J Am Dent Assoc 2015;146:830-9. [Crossref] [PubMed]
- Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, Bahrani-Mougeot FK. Bacteremia associated with toothbrushing and dental extraction. Circulation 2008;117:3118-25. [Crossref] [PubMed]