Anatomic techniques for cervical pedicle screw placement
Introduction
Posterior instrumentation of the cervical spine with pedicle screws has long been considered risky given the low margin of error that it affords. Cervical pedicle screw (CPS) breach rates have been reported as being anywhere from 1.1–29.0% (1-5). The possible neurovascular complications including injury to the nerve roots as well as the vertebral artery (3,6,7) are daunting to even the most experienced surgeons. Anatomical variations in cervical pedicles as well as the course of the vertebral arteries contribute to the challenge (8). However, studies have shown that the use of CPS results in biomechanically stronger fixation constructs than the use of lateral mass screws (9-13). As such, fixation with cervical pedicles screws is advantageous for deformity surgery (14-16), osteoporotic bone (17), and stabilization of unstable segments such as in trauma, tumour and spondyloarthritis (18-20).
In view of the aforementioned risk-benefit profile, there have been many endeavours to reduce the margin of error during insertion of CPS—these include detailed anatomical studies (21-25), drill jig template designs (26-33), as well as the use of navigation (10,34-43). Despite these numerous studies, at present, there has yet to be an established gold standard technique for insertion of CPS. While 3D printing (of patient-specific drill jigs), navigation and robotic technologies continue to develop and help narrow the margin for error, expertise in freehand insertion of CPS remains pertinent. It is only with a good understanding of the freehand technique that we can fully utilize technology to improve outcomes. Therefore, this review article aims to discuss freehand CPS insertion techniques as established in the current literature, while sharing experience from the senior author who exclusively performs pedicle screw insertion for posterior cervical instrumentation.
Methodology
A comprehensive literature search was performed for this review study using the following electronic databases: PubMed, Medline, EMBASE, Web of Science, Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews on the 22nd of July 2019. Search terms used were a combination of “cervical spine’’, “pedicle screws”, “insertion’’, and “techniques’’. The reference lists of included studies and review articles identified were also hand-searched for additional eligible articles. We included all full-text articles on CPS insertion techniques, focusing on clinical studies and those describing freehand techniques. Articles which were on cadaveric studies, drill jig, navigation or robotic technology were excluded. Other exclusion criteria meta-analyses and non-English publications.
Results
Study characteristics
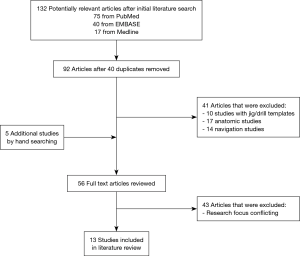
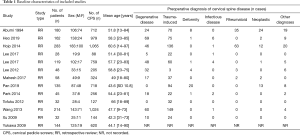
The search strategy identified 132 potentially relevant studies; titles were de-duplicated, and abstracts were screened. A further 43 studies were excluded due to conflicting research focus after review of 56 full-text articles (Figure 1). In all, a total of 13 primary references comprising 1,480 patients were included in this review (1,2,20,44-53). Baseline characteristics of included studies and study population are detailed in Table 1.
Full table
Surgical techniques
Entry point landmarks
The present study finds significant heterogeneity in reporting of landmarks for CPS insertion across all 13 studies. Of note, majority of studies reported (to varying levels of detail) utilizing the cranial margin of lamina for C2 levels, as well as lateral to centre of the articular mass, and just medial to the lateral border of the superior articular process for C3–7 levels. A summary of various reported landmarks for CPS insertion are noted in Table 2. Pilot hole formation was primarily performed via removal of cortical bone with high-speed burrs with head types ranging from 1.8 to 4 mm.
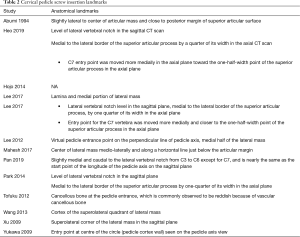
Full table
Tracking and formation of trajectory
Eight of 13 included studies utilized curved pedicle probes (tips ranging from 2 to 2.5 mm) to track through the pilot hole(1,2,44,46,48,49,52,53), with varying insertion depths ranging from specific measurements (20–30 mm) to ratioed measurements (no less than two-thirds of lateral mass thickness) under fluoroscopic guidance (46,48). Alternatively, nerve retractors (20), or a 2 mm burr (47) were used instead for path-tracking. Three studies specified the method for locating and subsiding into the cancellous channel by pushing the pedicle probe medially whilst in contact with the thick medial cortical pedicle wall, with an upward and downward movement of the tip (1,45,49). Subsequently, ball-tip probes were reported in most included studies to facilitate finding the right trajectory, with straight probes ensuring a wider, straighter track (1,45).
Limited included studies reported trajectory of CPS insertion in detail. In total, 7 studies noted the angle of screw insertion, with only Xu et al. accounting for superior-inferior trajectory (10° cephalad for C6 and 7, 10° caudad for C3 and C4, and vertically for C5) (52). Of note, other studies exhibited convergence angle trajectory ranging from 30° to 47° between the sagittal plane and longitudinal axis in the middle cervical spine (2,20,47,51,53).
Pedicle wall integrity and screw insertion
Seven studies specified use of a ball-tip probe at various checkpoints to confirm pedicle wall integrity, namely after insertion of curved probes, insertion of straight probes, and after tapping (1,2,45,47,48,51,52). Detection of perforation or screw malposition was followed by pedicle screw repositioning or conversion to lateral screw mass. A total of 10 studies reported subsequent tapping with 3.5 mm diameter tap followed by insertion of screw diameters ranging from 3.5–4.0 mm (1,2,44,47-53).
Discussion
As the margin of error in CPS fixation is small, meticulous planning of the surgery is crucial. The main risks associated with this surgery, such as vertebral artery injury, nerve root injury, CSF leak and postoperative infection (as detailed in Table 3), can be minimized with sufficient analysis of pre-operative imaging, the surgeon’s expertise and thorough knowledge of the local anatomy, as well as the use of validated, reproducible techniques of screw placement.
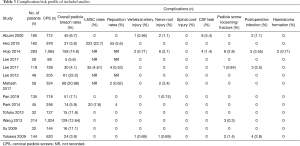
Full table
Pre-operative imaging
Pre-operative imaging in the form of a CT scan of the cervical spine is essential for the insertion of CPS. To begin with, it is important to determine from the CT scan whether the pedicle of interest can accommodate a screw. Previous cervical pedicle morphometric studies have shown that the outer pedicle diameter is more than 5 mm in most cervical vertebrae (54-56). CPS diameters used are usually 3.5, 4.0 and 4.5 mm, while screw lengths are usually 20, 22, and 24 mm (57). An outer pedicle diameter less than 4 mm makes CPS insertion challenging, and alternative forms of fixation such as lateral mass screws or posterior wiring can be considered. CPS are also contraindicated if the medullary canal of the pedicle appears to be sclerotic on pre-operative radiographic images as this confers a higher risk of screw malposition (8). Spine surgeons should meticulously evaluate the CT images pre-operatively in order to check the shape, size and direction of the pedicle, as well as to identify the screw trajectory and entry point. We recommend fine cut axial CT scans in 1.0–2.0 mm slices for this purpose. For each vertebra, right and left pedicles should be individually analyzed as pedicle morphology can often vary within a single vertebra. Usually, a smaller pedicle diameter accompanies an enlarged transverse foramen, signifying that the vertebral artery coursing through it is dominant on that side (58-60). In one study of 127 subjects, left vertebral artery dominance was 69.3% (61).
Some surgeons routinely perform 4-vessel MRI angiograms pre-operatively to study the course of the patient’s vertebral arteries. Others only perform an angiogram when there is a high index of suspicion (for example, if the aforementioned transverse foramen variations are noted on CT), to look for meandering or aberrancies in the vertebral arteries’ course. The vertebral arteries are known to sometimes loop close towards the vertebral bodies (8), and either an irregular vertebral artery pathway or irregular anatomy of the cervical pedicle can be present in up to 23.6% of patients (61). The highest incidence of irregularities is found at C2 (61), and therefore, some spine surgeons request for MRI angiogram whenever instrumentation of C2 and above is involved. Unless there is an intraosseous vertebral artery course, keeping the screw within the pedicle and vertebral body is always safe, and should be emphasized. A morphometric study by Tomasino et al. demonstrated significant individual variations in pedicle diameter, safe zone, and space occupied by the vertebral artery in the transverse foramen (61). These findings highlight the importance of evaluating the potential risks to the neurovascular structures prior to inserting CPSs.
Operation room set-up and positioning
As per Abumi et al. (19,20,57), we also recommend that the first surgeon stand at the head of the patient to be able to determine optimal trajectory for bilateral screw placement. The assistant usually stands at the left side of the patient next to the C-arm. The patient is positioned prone on the surgical table with his/her head secured firmly with a skull clamp to provide 3-point rigid cranial fixation. If the lower cervical levels are to be visualized, the shoulders are pulled caudally with heavy adhesive bandages to ensure ease of lateral fluoroscopic imaging.
Exposure
We recommend a generous longitudinal skin incision for CPS insertion to better determine and visualize the entry point, as well as to achieve the necessary screw convergence angle. The cranially adjacent lamina and lateral masses of the upper instrumented vertebra should be almost entirely exposed, taking care to preserve the cranial facet joint capsule and interspinous ligament. The paravertebral muscles are dissected laterally for complete exposure of the lateral margins of the articular masses. This helps determine screw entry point, as will be described below.
Implant selection
Screws should be selected according to each pedicle width and length. Based on the senior author’s experience, diameters of 3.5 to 4.5 mm are appropriate, and lengths around 22 to 30 mm are often used for C3–C7 vertebrae. The use of polyaxial screws is recommended, unless there is a need to correct for rotational deformity.
Entry point landmarks
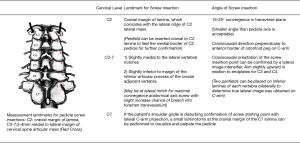
Abumi et al. were successful in establishing generic entry points to guide the placement of CPS (8), and till present, their methods are emulated by many spine surgeons. They recommended using the lateral vertebral notches of cervical vertebrae as a reference point to identify the entry points for C2 through to C7. Figure 2 combines the recommendations by Abumi et al. and illustrations by the senior author.
Pan et al. also recognized the anatomic consistency of the lateral vertebral notch as first described by Abumi et al. (20) and conducted radiological (62), cadaveric (63) and clinical (48) studies to demonstrate that using the lateral vertebral notch as a reference point to determine CPS entry points is a safe and accurate technique (48). However, the offsets from the lateral vertebral notch described by Pan et al. (48) differ marginally from Abumi et al. (8).
While it is helpful to use a given set of landmarks, such as the aforementioned, as an initial guide during pre-operative planning, these should not be prescriptive. Rather, we advocate that patient-specific, level-specific, and side-specific entry points should be identified on pre-op CT and confirmed on intraoperative image intensifier C-arm. Furthermore, in cases involving markedly degenerative lateral masses, the identification of prescribed entry points may prove difficult. Use of a high-speed burr to remove the proliferative lateral mass may help reveal more familiar anatomy, and help determine a tenable entry point. Hojo et al. evaluated over 1,000 screws with CT and found that malposition rate of CPS inserted by freehand technique was especially high (26.7%) in rheumatoid patients and attributed this to the difficulty in finding the established screw entry points due to destructive changes in the posterior elements as well as severe bone fragility (2). This finding reiterates the importance of identifying patient-specific screw trajectories and entry points on the pre-operative CT prior to insertion of CPS. In the senior author’s own series, patients with previous anterior cervical spine fusion surgeries, cervical scoliosis with rotational deformities and ankylosed spines are at a higher risk of screw malpositioning.
Screw insertion
After adequate dissection, the lateral C-arm is used for localization of the entry point during screw insertion and should remain in position for the duration of insertion.
In terms of superior-inferior trajectory, Abumi et al. recommend that the angle of screw insertion in the sagittal plane should be parallel to the cranial endplate for C5 to C7 pedicles, while aiming slightly cephalad for C2 to C4 pedicles (8,57).
In terms of convergence angle trajectory, anatomical studies have shown that the average angle between the sagittal plane and the longitudinal pedicle axis in the middle cervical spine was 46° (range, 30–62°) (64). The convergence angles generally increase down the cervical spine, with the smallest angle occurring at C2, and the largest angle occurring at C5 (64,65).
Yukawa et al. introduced their technique of finding the “pedicle axis view” to determine the appropriate screw trajectory (7,53). In this technique, a true lateral view of the vertebra is obtained, followed by the fluoroscope being turned until it shows the cortex of the pedicle of interest appearing as a circle – the “pedicle axis view”.
The angle at which the fluoroscope is tilted to obtain this view gives the trajectory in which to insert the screw. Unlike Abumi et al., Yukawa et al. did not utilize the lateral vertebral notch to determine entry point and did so instead by correlating the true lateral and pedicle axis views. Therefore, while they reported finding the convergence angle to range from 30 to 55°, they mentioned their preference to for a smaller screw trajectory (30–35°) in order to prevent the need for additional surgical exposure (7).
Abumi et al. also described a funnel technique, in which, using a diamond-tipped high-speed burr, a funnel-like whole is created at the screw entry point, down to the beginning of the pedicle. The funnel created allows direct visualization of the pedicle, and also allows for wider range of trajectories in which the screw can be inserted (8). This method is thus versatile, while reducing the risk of vertebral artery injury. With this technique, the aim is to insert a screw in an oblique angle smaller than the angle of the true anatomical axis of the pedicle. A convergence trajectory of 40–60° from the sagittal plane for C3 to C6 pedicles is desirable, while a smaller convergence trajectory is required for C2 and C7 pedicles (57).
As with the rest of the spine, the medial cortex of the cervical pedicle is thicker than that of the lateral (55). Therefore, the medial pedicle must be used as a guide to insert a screw into the vertebral body through the pedicle isthmus, and tactile awareness of the medial cortex in contrast to the lateral cancellous bone is important during probing, tapping and screw insertion. Some groups have tried to exploit this by coming up with their own varying methods such as the “medial funnel technique” (44) and the “cervical medial cortical pedicle screw” (66).
Prior to insertion of the screws, we recommend that the surgeon confirms the direction and insertion depth via a small ball-tip probe visualized on lateral II. Heo et al. laid out 5 key steps for safe CPS insertion (1):
- Screw entry point determination on preoperative CT scan;
- Small, curved pedicle probe used to ensure sufficient medial angle for screw insertion;
- Detection of pedicle breach using a ball-tip probe;
- Conversion to a lateral mass screw when a breach is detected;
- Check on the intraoperative AP radiograph for acceptable screw position.
With regards to Heo et al.’s steps (II) and (III), if a large amount of venous bleeding is encountered, it is highly suggestive of breach, as the bleeding may originate from either the venous plexus around the vertebral artery or the venous plexus within the vertebral canal itself.
Karaikovic et al. suggested that there is no safe zone anterior to the cervical vertebral bodies in the cervical spine except at the level of C2 (67). Thus, they recommended that only C2 allows for bicortical purchase of pedicle screws. However, in the senior author’s series, bicortical screws were placed for all levels to allow for stronger fixation, and a penetration of 1 mm of the anterior cortex remained safe. Nevertheless, bicortical screw purchase in the cervical spine is not always necessary, and its biomechanical advantages should be weighed against the known potential complications and the individual surgeon’s expertise in CPS placement.
Given the challenges faced in inserting CPS, there have been numerous other publications describing various other methods of identifying the ideal entry point or screw trajectory. Besides detailed studies performed to validate drill jig template designs (26-33), as well as the use of navigation (10,34-43) there have been other studies identifying anatomical references in attempts to mitigate risk of screw malposition. These include the superomedial edge of lamina (68), the nutrient foramen (25), and intersection between the horizontal line through the midpoint of the transverse process root and the vertical line through the mediolateral third of the superior articular process (69) to locate the entry point; as well as the contralateral lamina (70) and ipsilateral lamina pedicle angle (21) to aim the screw in the right trajectory. However, with the exception of navigation, the aforementioned specific methods have not gained widespread practice.
Learning curve
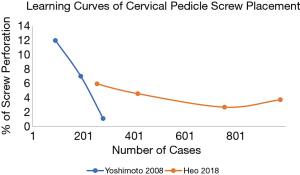
Given the challenges faced during CPS insertion, it is important that we recognize its significantly steep learning curve so that spine surgeons learning the technique can receive the appropriate guidance necessary to minimize the possibility of making fatal mistakes. Heo et al. reported an initial 6% breach rate, and a plateaued breach rate of 3% (1), while Yoshimoto et al. reported an initial 12% breach rate, and a plateaued breach rate of 1% (5). Figure 3 superimposes the learning curves of the two studies for comparison.
Conclusions
Instrumentation with CPS is a worthwhile endeavour despite its inherent risks as CPS confer biomechanical advantage useful in certain clinical scenarios. To date, there is no single gold standard technique for CPS insertion. While Abumi’s pioneering technique remains the most well-known method of freehand pedicle screw insertion, newly emerged adaptations, and complementary techniques further improve the practicality of this mode of posterior instrumentation. Ultimately, success in CPS insertion is dependent on the surgeon's ability to identify the ideal screw entry point and trajectory—both of which are made less elusive with meticulous preoperative planning. Therefore, while work on patient-specific drill jigs, navigation and robotic technologies has brought significant progress to the field and improved the safety profile of this procedure, good expertise in freehand CPS insertion technique remains all the more pertinent.
Acknowledgments
Professor Kuniyoshi Abumi for his inspirational teachings in cervical pedicle screw insertion that resulted in the senior author’s passion for this field of spine surgery
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lee A. Tan and Ilyas S. Aleem) for the series “Advanced Techniques in Complex Cervical Spine Surgery” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: The series “Advanced Techniques in Complex Cervical Spine Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Heo Y, Lee SB, Lee BJ, et al. The Learning Curve of Subaxial Cervical Pedicle Screw Placement: How Can We Avoid Neurovascular Complications in the Initial Period? Oper Neurosurg (Hagerstown) 2019;17:603-7. [Crossref] [PubMed]
- Hojo Y, Ito M, Suda K, et al. A multicenter study on accuracy and complications of freehand placement of cervical pedicle screws under lateral fluoroscopy in different pathological conditions: CT-based evaluation of more than 1,000 screws. Eur Spine J 2014;23:2166-74. [Crossref] [PubMed]
- Abumi K, Shono Y, Ito M, et al. Complications of Pedicle Screw Fixation in Reconstructive Surgery of the Cervical Spine. Spine 2000;25:962-9. [Crossref] [PubMed]
- Neo M, Sakamoto T, Fujibayashi S, et al. The Clinical Risk of Vertebral Artery Injury From Cervical Pedicle Screws Inserted in Degenerative Vertebrae. Spine 2005;30:2800-5. [Crossref] [PubMed]
- Yoshimoto H, Sato S, Hyakumachi T, et al. Clinical accuracy of cervical pedicle screw insertion using lateral fluoroscopy: a radiographic analysis of the learning curve. Eur Spine J 2009;18:1326-34. [Crossref] [PubMed]
- Kast E, Mohr K, Richter HP, et al. Complications of transpedicular screw fixation in the cervical spine. Eur Spine J 2006;15:327-34. [Crossref] [PubMed]
- Yukawa Y, Kato F, Yoshihara H, et al. Cervical pedicle screw fixation in 100 cases of unstable cervical injuries: pedicle axis views obtained using fluoroscopy. J Neurosurg Spine 2006;5:488-93. [Crossref] [PubMed]
- Abumi K, Ito M, Sudo H. Reconstruction of the subaxial cervical spine using pedicle screw instrumentation. Spine (Phila Pa 1976) 2012;37:E349-56. [Crossref] [PubMed]
- Johnston TL, Karaikovic EE, Lautenschlager EP, et al. Cervical pedicle screws vs. lateral mass screws: uniplanar fatigue analysis and residual pullout strengths. Spine J 2006;6:667-72. [Crossref] [PubMed]
- Kotani Y, Abumi K, Ito M, et al. Improved accuracy of computer-assisted cervical pedicle screw insertion. J Neurosurg 2003;99:257-63. [PubMed]
- Kothe R, Rüther W, Schneider E, et al. Biomechanical Analysis of Transpedicular Screw Fixation in the Subaxial Cervical Spine. Spine 2004;29:1869-75. [Crossref] [PubMed]
- Schmidt R, Wilke HJ, Claes L, et al. Pedicle Screws Enhance Primary Stability in Multilevel Cervical Corpectomies: Biomechanical In Vitro Comparison of Different Implants Including Constrained and Nonconstrained Posterior Instumentations. Spine 2003;28:1821-8. [Crossref] [PubMed]
- Ito Z, Higashino K, Kato S, et al. Pedicle Screws Can be 4 Times Stronger Than Lateral Mass Screws for Insertion in the Midcervical Spine. J Spinal Disord Tech 2014;27:80-5. [Crossref] [PubMed]
- Abumi K, Shono Y, Taneichi H, et al. Correction of Cervical Kyphosis Using Pedicle Screw Fixation Systems. Spine 1999;24:2389. [Crossref] [PubMed]
- Abumi K, Takada T, Shono Y, et al. Posterior Occipitocervical Reconstruction Using Cervical Pedicle Screws and Plate–Rod Systems. Spine 1999;24:1425. [Crossref] [PubMed]
- Rajasekaran S, Kanna PR, Shetty TA, et al. Intra-operative computer navigation guided cervical pedicle screw insertion in thirty-three complex cervical spine deformities. J Craniovertebr Junction Spine 2010;1:38-43. [Crossref] [PubMed]
- Abumi K, Kaneda K, Shono Y, et al. One-stage posterior decompression and reconstruction of the cervical spine by using pedicle screw fixation systems. J Neurosurg 1999;90:19-26. [Crossref] [PubMed]
- Abumi K, Kaneda K. Pedicle Screw Fixation for Nontraumatic Lesions of the Cervical Spine. Spine 1997;22:1853-63. [Crossref] [PubMed]
- Abumi K, Ito M, Kaneda K. Surgical Treatment of Cervical Destructive Spondyloarthropathy (DSA). Spine 2000;25:2899-905. [Crossref] [PubMed]
- Abumi K, Itoh H, Taneichi H, et al. Transpedicular Screw Fixation for Traumatic Lesions of the Middle and Lower Cervical Spine. J Spinal Disord 1994;7:19-28. [Crossref] [PubMed]
- Bayley E, Zia Z, Kerslake R, et al. The ipsilateral lamina-pedicle angle: can it be used to guide pedicle screw placement in the sub-axial cervical spine? Eur Spine J 2010;19:458-63. [Crossref] [PubMed]
- Lee DH, Lee SW, Kang SJ, et al. Optimal entry points and trajectories for cervical pedicle screw placement into subaxial cervical vertebrae. Eur Spine J 2011;20:905-11. [Crossref] [PubMed]
- Liu Y, Zhang B, Dai M, et al. Anatomic study of individualized and improved pedicle screw implantation in the lower cervical spine. Int Surg 2015;100:328-33. [Crossref] [PubMed]
- Munusamy T, Thien A, Anthony MG, et al. Computed tomographic morphometric analysis of cervical pedicles in a multi-ethnic Asian population and relevance to subaxial cervical pedicle screw fixation. Eur Spine J 2015;24:120-6. [Crossref] [PubMed]
- Oshina M, Oshima Y, Matsubayashi Y, et al. Nutrient foramen location on the laminae provides a landmark for pedicle screw entry: a cadaveric study. BMC Musculoskelet Disord 2018;19:293. [Crossref] [PubMed]
- Kashyap A, Kadur S, Mishra A, et al. Cervical pedicle screw guiding jig, an innovative solution. J Clin Orthop Trauma 2018;9:226-9. [Crossref] [PubMed]
- Lu S, Xu YQ, Lu WW, et al. A novel patient-specific navigational template for cervical pedicle screw placement. Spine (Phila Pa 1976) 2009;34:E959-66. [Crossref] [PubMed]
- Moser M, Farshad M, Farshad-Amacker NA, et al. Accuracy of Patient-Specific Template-Guided Versus Freehand Cervical Pedicle Screw Placement from C2 to C7: A Randomized Cadaveric Study. World Neurosurg 2019;126:e803-13. [Crossref] [PubMed]
- Owen BD, Christensen GE, Reinhardt JM, et al. Rapid prototype patient-specific drill template for cervical pedicle screw placement. Comput Aided Surg 2007;12:303-8. [Crossref] [PubMed]
- Ryken TC, Owen BD, Christensen GE, et al. Image-based drill templates for cervical pedicle screw placement. J Neurosurg Spine 2009;10:21-6. [Crossref] [PubMed]
- Yan J, Li K, Zheng Y, et al. The Ipsilateral Adjacent Laminae: A Reliable Guide in Determining the Direction of Subaxial Cervical Pedicle Axis in the Sagittal Plane. Spine (Phila Pa 1976) 2015;40:1647-52. [Crossref] [PubMed]
- Yu Z, Zhang G, Chen X, et al. Application of a novel 3D drill template for cervical pedicle screw tunnel design: a cadaveric study. Eur Spine J 2017;26:2348-56. [Crossref] [PubMed]
- Zhang G, Yu Z, Chen X, et al. Accurate placement of cervical pedicle screws using 3D-printed navigational templates: An improved technique with continuous image registration. Orthopade 2018;47:428-36. [Crossref] [PubMed]
- Bredow J, Oppermann J, Kraus B, et al. The accuracy of 3D fluoroscopy-navigated screw insertion in the upper and subaxial cervical spine. Eur Spine J 2015;24:2967-76. [Crossref] [PubMed]
- Chachan S, Bin Abd Razak HR, Loo WL, et al. Cervical pedicle screw instrumentation is more reliable with O-arm-based 3D navigation: analysis of cervical pedicle screw placement accuracy with O-arm-based 3D navigation. Eur Spine J 2018;27:2729-36. [Crossref] [PubMed]
- Ishikawa Y, Kanemura T, Yoshida G, et al. Clinical accuracy of three-dimensional fluoroscopy-based computer-assisted cervical pedicle screw placement: a retrospective comparative study of conventional versus computer-assisted cervical pedicle screw placement. J Neurosurg Spine 2010;13:606-11. [Crossref] [PubMed]
- Kantelhardt SR, Neulen A, Keric N, et al. Alternative radiation-free registration technique for image-guided pedicle screw placement in deformed cervico-thoracic segments. J Neurosurg Sci 2017;61:464-72. [PubMed]
- Liu YJ, Tian W, Liu B, et al. Comparison of the clinical accuracy of cervical (C2-C7) pedicle screw insertion assisted by fluoroscopy, computed tomography-based navigation, and intraoperative three-dimensional C-arm navigation. Chin Med J (Engl) 2010;123:2995-8. [PubMed]
- Rajasekaran S, Kanna PR, Shetty TA. Intra-operative computer navigation guided cervical pedicle screw insertion in thirty-three complex cervical spine deformities. J Craniovertebr Junction Spine 2010;1:38-43. [Crossref] [PubMed]
- Reinhold M, Bach C, Audige L, et al. Comparison of two novel fluoroscopy-based stereotactic methods for cervical pedicle screw placement and review of the literature. Eur Spine J 2008;17:564-75. [Crossref] [PubMed]
- Shimokawa N, Takami T. Surgical safety of cervical pedicle screw placement with computer navigation system. Neurosurg Rev 2017;40:251-8. [Crossref] [PubMed]
- Theologis AA, Burch S. Safety and Efficacy of Reconstruction of Complex Cervical Spine Pathology Using Pedicle Screws Inserted with Stealth Navigation and 3D Image-Guided (O-Arm) Technology. Spine (Phila Pa 1976) 2015;40:1397-406. [Crossref] [PubMed]
- Yoshii T, Hirai T, Sakai K, et al. Cervical pedicle screw placement using intraoperative computed tomography imaging with a mobile scanner gantry. Eur Spine J 2016;25:1690-7. [Crossref] [PubMed]
- Lee JH, Choi BK, Han IH, et al. Cervical Pedicle Screw Placement Using Medial Funnel Technique. Korean J Spine 2017;14:84-8. [Crossref] [PubMed]
- Lee S, Seo J, Lee MK, et al. Widening of the safe trajectory range during subaxial cervical pedicle screw placement: advantages of a curved pedicle probe and laterally located starting point without creating a funnel-shaped hole. J Neurosurg Spine 2017;27:150-7. [Crossref] [PubMed]
- Lee SH, Kim KT, Abumi K, et al. Cervical pedicle screw placement using the "key slot technique": the feasibility and learning curve. J Spinal Disord Tech 2012;25:415-21. [Crossref] [PubMed]
- Mahesh B, Upendra B, Vijay S, et al. Perforations and angulations of 324 cervical medial cortical pedicle screws: a possible guide to avoid lateral perforations with use of pedicle screws in lower cervical spine. Spine J 2017;17:457-65. [Crossref] [PubMed]
- Pan Z, Zhong J, Xie S, et al. Accuracy and Safety of Lateral Vertebral Notch-Referred Technique Used in Subaxial Cervical Pedicle Screw Placement. Oper Neurosurg (Hagerstown) 2019;17:52-60. [Crossref] [PubMed]
- Park JH, Jeon SR, Roh SW, et al. The safety and accuracy of freehand pedicle screw placement in the subaxial cervical spine: a series of 45 consecutive patients. Spine (Phila Pa 1976) 2014;39:280-5. [Crossref] [PubMed]
- Tofuku K, Koga H, Komiya S. Cervical pedicle screw insertion using a gutter entry point at the transitional area between the lateral mass and lamina. Eur Spine J 2012;21:353-8. [Crossref] [PubMed]
- Wang Y, Xie J, Yang Z, et al. Computed tomography assessment of lateral pedicle wall perforation by free-hand subaxial cervical pedicle screw placement. Arch Orthop Trauma Surg 2013;133:901-9. [Crossref] [PubMed]
- Xu RM, Ma WH, Wang Q, et al. A free-hand technique for pedicle screw placement in the lower cervical spine. Orthop Surg 2009;1:107-12. [Crossref] [PubMed]
- Yukawa Y, Kato F, Ito K, et al. Placement and complications of cervical pedicle screws in 144 cervical trauma patients using pedicle axis view techniques by fluoroscope. Eur Spine J 2009;18:1293-9. [Crossref] [PubMed]
- Patwardhan AR, Nemade PS, Bhosale SK, et al. Computed tomography-based morphometric analysis of cervical pedicles in Indian population: a pilot study to assess feasibility of transpedicular screw fixation. J Postgrad Med 2012;58:119-22. [Crossref] [PubMed]
- Karaikovic EE, Daubs MD, Madsen RW, et al. Morphologic characteristics of human cervical pedicles. Spine (Phila Pa 1976) 1997;22:493-500. [Crossref] [PubMed]
- Yusof MI, Ming LK, Abdullah MS, et al. Computerized tomographic measurement of the cervical pedicles diameter in a Malaysian population and the feasibility for transpedicular fixation. Spine (Phila Pa 1976) 2006;31:E221-4. [Crossref] [PubMed]
- Abumi K. Cervical spondylotic myelopathy: posterior decompression and pedicle screw fixation. Eur Spine J 2015;24 Suppl 2:186-96. [Crossref] [PubMed]
- Kotil K, Kilincer C. Sizes of the transverse foramina correlate with blood flow and dominance of vertebral arteries. Spine J 2014;14:933-7. [Crossref] [PubMed]
- O'Rahilly R, Müller F, Meyer DB. The human vertebral column at the end of the embryonic period proper. 2. The occipitocervical region. J Anat 1983;136:181-95. [PubMed]
- Gabrielsen TO. Size of vertebral artery and of foramen transversarium of axis. An anatomic study. Acta Radiol Diagn (Stockh) 1969;9:285-91. [PubMed]
- Tomasino A, Parikh K, Koller H, et al. The vertebral artery and the cervical pedicle: morphometric analysis of a critical neighborhood. J Neurosurg Spine 2010;13:52-60. [Crossref] [PubMed]
- Wu C, Huang Z, Pan Z, et al. Coronal Multiplane Reconstructed Computed Tomography Image Determining Lateral Vertebral Notch-Referred Pedicle Screw Entry Point in Subaxial Cervical Spine: A Preclinical Study. World Neurosurg 2017;103:322-9. [Crossref] [PubMed]
- Luo J, Wu C, Huang Z, et al. The accuracy of the lateral vertebral notch-referred pedicle screw insertion technique in subaxial cervical spine: a human cadaver study. Archives of Orthopaedic and Trauma Surgery 2017;137:517-22. [Crossref] [PubMed]
- Karaikovic EE, Yingsakmongkol W, Griffiths HJ, et al. Possible Complications of Anterior Perforation of the Vertebral Body Using Cervical Pedicle Screws. J Spinal Disord Tech 2002;15:75-8. [Crossref] [PubMed]
- Reinhold M, Magerl F, Rieger M, et al. Cervical pedicle screw placement: feasibility and accuracy of two new insertion techniques based on morphometric data. Eur Spine J 2007;16:47-56. [Crossref] [PubMed]
- Mahesh B, Upendra B, Mahan RS. The medial cortical pedicle screw--a new technique for cervical pedicle screw placement with partial drilling of medial cortex. Spine J 2014;14:371-80. [Crossref] [PubMed]
- Karaikovic EE, Yingsakmongkol W, Gaines RW Jr. Accuracy of cervical pedicle screw placement using the funnel technique. Spine (Phila Pa 1976) 2001;26:2456-62. [Crossref] [PubMed]
- Nishizawa K, Mori K, Nakamura A, et al. Novel Landmark for Cervical Pedicle Screw Insertion Point from Computed Tomography-Based Study. Asian Spine J 2017;11:82-7. [Crossref] [PubMed]
- Liu J, Li Y, Wu Y, et al. A novel method of cervical pedicle screw placement from C3 to C5 and its clinical applications. Spine (Phila Pa 1976) 2013;38:E504-12. [Crossref] [PubMed]
- Hacker AG, Molloy S, Bernard J. The contralateral lamina: a reliable guide in subaxial, cervical pedicle screw placement. Eur Spine J 2008;17:1457-61. [Crossref] [PubMed]