Clinical outcomes for lumbar fusion using silicon nitride versus other biomaterials
Introduction
Lumbar spinal fusion is typically performed for intractable low-back pain (LBP) or radicular symptoms after failure of extended conservative treatments (1). Symptoms can result from degenerative intervertebral disc disease (DDD), disc herniation, stenosis, vertebral endplate sclerosis, osteophytes, spondylolisthesis, spondylosis, scoliosis, infection, tumors, or trauma (2-4). A central lumen in the intervertebral spacers holds bone graft, while the implant provides segmental stability and restores disc height, lordotic curvature, and sagittal balance (5,6).
Spacer design and biomaterials have evolved over the past 30 years (7). Early use of structural iliac crest autogenous bone graft was limited by donor site morbidity, leading to interest in allograft and synthetic biomaterials. Today, polyetheretherketone (PEEK), titanium (Ti), tantalum (Ta), and silicon nitride (Si3N4) reflect biomaterial choices for cage manufacture (8,9). Of these, Si3N4 has the longest clinical history. It was originally implanted in patients who underwent lumbar fusion beginning in 1986 (10). Despite design limitations, those early Si3N4 implants proved to be safe and efficacious at thirty years of follow-up (11). The U.S. FDA cleared Si3N4 intervertebral spinal spacers in 2008, followed by the European Union in 2009, Brazil in 2015, and Australia in 2017. To date, over 35,000 Si3N4 spinal fusion devices have been implanted, with <0.07% reportable adverse events (SINTX Technologies, Inc., 2019, unpublished data).
Because of a paucity of prior clinical results, the purpose of this study was to augment existing data with a significant retrospective review of lumbar fusion outcomes using Si3N4 cages (450 patients, 519 implants) at four U.S. centers. Preoperative patient demographics, pain scores, comorbidity data along with post-operative last follow-up pain scores, complications, adverse events, and secondary surgical interventions (SSI) were obtained from chart reviews of 450 patients who received Si3N4 implants. Results were compared to lumbar fusion reported with other biomaterials in 26 cohorts comprised of 1,025 patients, reported in 14 publications. The null hypothesis was that Si3N4 lumbar fusion outcomes would not be different from those reported in the control group.
Methods
Review of medical records
In accordance with the study protocol, an experienced medical records examiner was independently contracted to retrieve data from the charts of all patients who received a Si3N4 lumbar fusion implant by four surgeons at different medical centers between November 2017 and June 2018. Although IRB approval was not required for this study, patient information and data remained anonymous and in compliance with IRB standards. Inclusion criteria are listed in Table 1. There were no exclusion criteria. Data were recorded from both digital and active or archival hard copy files.

Full table
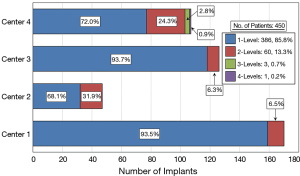
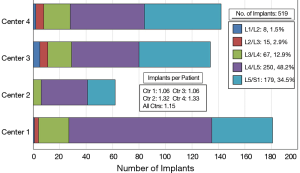
Several different surgical approaches were utilized including anterior lumbar interbody fusion (ALIF), transforaminal lumbar interbody fusion (TLIF), and posterior lateral interbody fusion (PLIF). Two generations of Si3N4 spacers were used in this study (Figure 1). Figure 2 provides a breakdown of the number of single and multilevel surgeries by center. Over 85% of the patients were operated on at one level and about 13% at two levels. Three and four level procedures were a rarity at <1.0%. A total of 519 Si3N4 devices were implanted as shown in Figure 3, with over 80% of the implantations occurring between L4 and S1. Of this total, approximately 59.5% were PLIF, 37.4% TLIF, and 3.1% ALIF implants.

Surgical procedures
The surgical procedure varied based on the approach and implant type chosen by the surgeon (12). For PLIF, patients were placed in a prone position and either an open midline incision with bilateral muscle dissection or a MIS paramedian muscle splitting incision was used to access the posterior vertebral column. A laminectomy was generally performed based on surgeon preference and the dura retracted to expose the disc space. A complete discectomy was performed followed by endplate preparation. A sizer was used to determine the appropriate height, width, depth, and lordosis of the intervertebral space. Based on these measurements, a Si3N4 cage was selected. Local osteophytes that were removed during endplate preparation were morselized and, along with burr shavings, packed into the lumen of the implant. Demineralized bone matrix (DBM) was added based on surgeon preference. After placement of the interbody device, bilateral pedicle screws and rods were inserted into the superior and inferior segments for added stabilization. For TLIF, the patients were also placed in a prone position and a midline or paramedian incision was conducted. A unilateral laminectomy and inferior facetectomy were then performed to expose the spinal canal followed by removal of the natural disc and endplate preparation. The remaining operative steps were similar to the PLIF approach. For ALIF (13), patients were placed in the supine position. This anterior approach involved midline, paramedian, or minimally invasive incisions to split the oblique abdominal muscle followed by retraction of retroperitoneal organs and vasculature to form a corridor to the spinal column. The technique provided a direct view of disc space and lateral exposure of the vertebral segments which permitted removal of the disc and endplate preparation. Appropriately sized Si3N4 implants, packed with morselized bone and DBM, were then placed in the disc space. A subsequent posterior operative procedure was used to place bilateral pedicle screws and rods for added stabilization. Patients were mobilized soon after surgery without orthoses. Upon discharge, they were instructed to restrict bending, twisting and lifting efforts during the recovery period of no more than ~11.3 kg (25 lbs.) for between 6 and 12 weeks.
Data acquisition
Each patient’s preoperative demographic data (age, gender, height, weight, BMI, and diagnoses), comorbidity conditions (smoking, diabetes, hypertension, osteoporosis, osteopenia, tumor, and other), along with their post-operative results (days to last follow-up, pain scores, complications, adverse events, and SSIs) were extracted from their respective medical charts. Pain scores were assessed using the visual analog scale (VAS), zero being “no pain” and ten being the “worst pain imaginable”. Pain scores were taken as the maximum of either back, leg, or bodily pain at each follow-up visit. For consistency with the metadata, scores were converted to a zero to 100-point scale. Complications and adverse events included recurrent symptoms, adjacent level disease (ALD), subsidence, infection, migration or non-union, and hematoma. SSIs were compiled for patients experiencing ALD and pain associated with pedicle screw position or removal.
Meta-analysis
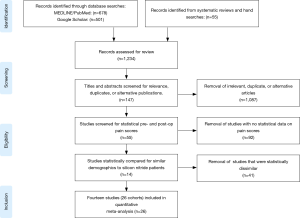
A meta-analysis was performed to quantitatively assess and compare differential changes in pain scores, complications, adverse events, and SSI for patients implanted with Si3N4 cages versus other commonly used lumbar interbody devices. MEDLINE/PubMed was searched for relevant publications using a human clinical query with the search terms of “(Lumbar Spinal Fusion) AND (Pain) AND (VAS)” along with filters for years (2000 to 2019), abstract and full text in English, and Adults (≥19 years of age). The output was augmented by a Google Scholar search with the added terms of “(Standard Deviation) OR (Confidence Interval)”. Article titles and abstracts were then compared, and duplicates removed. Additional clinical papers were identified from a number of published systematic reviews and meta-analyses (14-17) and by manual searches. Papers were excluded if the reported studies were for follow-up periods of <6 months or if they lacked quantifiable statistical data for pre-op and follow-up pain scores. Of the remaining articles, those selected for inclusion had statistically similar pre-op demographics. Fourteen studies consisting of 26 cohorts and 1,025 patients were selected (18-31). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for included articles is shown in Figure 4 (32).
Statistical analysis
Statistical analyses including metadata comparisons were performed using MedCalc Ver. 18.6-64 bit (Ostend, Belgium). Ordinal data were analyzed using Student’s t-tests whereas nominal results used proportionality assessments including Chi-squared and Fisher’s exact tests. Significance was set at P values of <0.05. An independent statistician (Biomedical Statistical Consulting, Wynnewood, PA USA) assisted in performing the meta-analysis.
Results
Preoperative diagnosis, demographics, and comorbidities
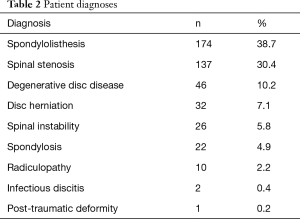
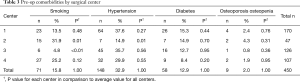
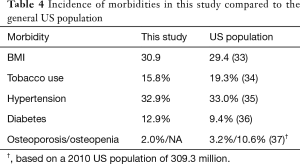
Four lumbar spine disorders (spondylolisthesis, spinal stenosis, degenerative disc disease, and disc herniation) accounted for over 85% of patient diagnoses. The entire etiological data are provided in Table 2. Of the 450 patient records included in the study, the average age was 58.2±12.4 years, 56.2% were female, and the average BMI score was 30.9±6.1. There were no statistical gender differences between the four surgical centers and only center 4 had a slightly younger population (55.5 years, P=0.04). Pre-op comorbidities are presented in Table 3. The patient count in this and subsequent tables or charts do not total to the original enrollment due to the fact that some data were missing from patients’ records. Patients from all four centers bordered on being clinically obese. However, centers 1 and 3 were at opposite ends of the statistical spectrum (32.2, P=0.02, and 29.4, P=0.01, respectively) when compared to the average of all four centers. For pre-op comorbidities, Table 3 shows that 15.8% of the patients were smokers, 32.9% had high blood pressure, 12.9% were diabetic, and 2.0% were diagnosed with osteoporosis or osteopenia. Center 2 had the highest percentage of smokers (31.9%, P=0.01) whereas center 3 had the fewest (4.8%, P<0.01). While nearly one-third of all patients were hypertensive, center 2 had the smallest statistical proportion of patients with this morbidity (i.e., 14.9%, P=0.01). There were no statistical differences between the centers for patients with diabetes or osteoporosis/osteopenia. Heterogeneity tests conducted for differences in these demographic and pre-op comorbidities showed homogeneous statistics for gender (I2=45.8%, P=0.14), diabetes (I2=4.1%, P=0.37), and osteoporosis/osteopenia (I2=0.0%, P=0.52), whereas heterogeneous values were observed for age (I2=60.9%, P=0.05), BMI (I2=76.3%, P<0.01), smoking (I2=90.2%, P<0.01), and hypertension (I2=71.9%, P=0.01). However, the incidence of these morbidities was fairly representative of the greater US population as shown in Table 4.
Full table
Full table
Full table
Clinical outcomes
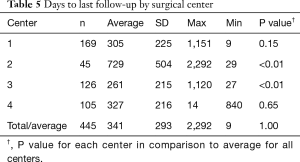
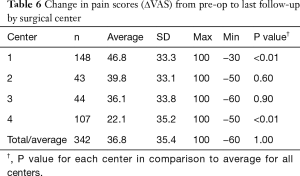
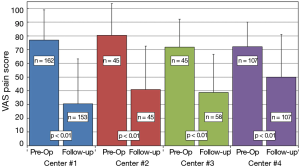
Average time to last-follow-up for each of the four surgical centers is presented in Table 5. Significant differences were noted in last follow-up periods with center 3 having the shortest period (261±215 days, 8.7±7.2 months) and center 2 having the longest (729±504 days, or 24.3±16.8 months). The overall longest follow-up also occurred for center 2 at 2,292 days (~6.4 y). Clinical results for changes in VAS pain scores for the four centers are provided in Table 6. Patients from each center experienced significant reductions in VAS pain scores (P<0.01) from pre-op to last follow-up. A summary of VAS pain scores for each center along with their statistical significance is shown in Figure 5. Patients from center 1 had the largest reductions in pain (46.8 points) with patients from center 4 showing the smallest change (22.1 points). Overall, 77.6% of patients reported an improvement in their pain scores at ≤2 years follow-up, with 69.0% showing ≥25-point improvement and 60.0% indicating an improvement of more than 35-points. Between the four centers these results were homogeneous for pre-op pain scores (I2=51.2%, P=0.11) but heterogeneous for last follow-up (I2=83.8%, P<0.01) and ΔVAS (I2=86.7%, P<0.01) pain scores.
Full table
Full table
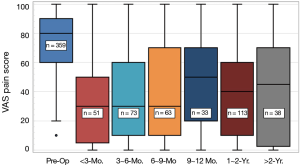
Box and whisker plots for pain scores are provided in Figure 6 as a function of last follow-up. The largest reduction in pain occurred in the post-operative periods up to nine-months. Mean values dropped from 75.3-point for pre-op to 34.9-, 36.7-, and 38.4-point for the periods of <3, 3–6, and 6–9 months, respectively. Thereafter, average pain scores moderately increased for the remaining patients at 1-year (45.8-point) but declined at 1–2 years (37.9-point) and increased at >2 years (42.6-point). However, they never returned to their pre-op levels. Covariant analyses were performed to assess the effects of demographics and pre-op comorbidities on follow-up pain scores. In comparing data for follow-up periods of <9 to >9 months, it was discovered that there was a significantly higher proportion of patients with osteoporosis/osteopenia for patients with >9 months follow-up (i.e., 4.05% versus 0.448%, P=0.01). Poor bone quality may have been a contributing factor to the increased pain scores for the later follow-up periods because these patients showed higher last follow-up pain values (i.e., 55.0±32.1 points, n=8, P=0.15) than the average of the four centers. Eleven of the 44 total complications and one SSI for persistent pain due to a pedicle screw were also associated with follow-up periods >12 months. There were no other covariant factors that had statistically significant contributions to the higher pain scores for the later follow-up periods.
Meta-analysis
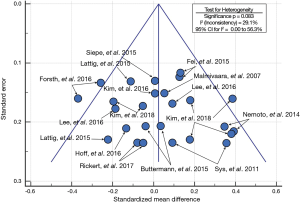
Metadata from fourteen lumbar fusion studies comprising of 26 cohorts and 1,025 patients were selected as the control group for comparison to the Si3N4 results. Although a larger number of studies were initially considered for inclusion, only 14 met similar demographic criteria. Most studies had much younger patient populations (typically >10-year differential). The 14 studies were composed of a mixture of single- and multicenter randomized controlled trials (RCTs) and retrospective or observational studies assessing the effectiveness of various lumbar fusion methods using a range of commonly accepted spacers or cages and surgical approaches. Details of the selected studies are provided in Table 7. Table 8 compares the pre-op demographics of the Si3N4 group to the compiled metadata. There were no statistical differences in either age or gender between the two groups but the Si3N4 cohort had higher BMI values than the control (30.9 versus 25.8, P<0.01). In fact, it was difficult to locate comparative studies that matched this comorbidity. Of the 55 studies selected for final review (cf., Figure 4), only four cohorts had statistically similar BMI data, and from them, only one had common age and gender statistics. In contrast, patients from the Si3N4 group included a lower number of smokers than the control (15.8% versus 30.0%, P<0.01). Table 9 provides clinical outcomes for both the Si3N4 and metadata groups in terms of changes in VAS pain scores, complications, adverse events, and SSI. There were no statistical differences in any of these comparative measures. A heterogeneity test for the metadata is provided in the funnel plot of Figure 7. This test compares mean and 95% confidence intervals for changes in VAS pain scores for the 26 meta-analysis cohorts to the mean and pooled 95% confidence interval from this Si3N4 study. The test indicates reasonable homogeneity of the data (I2=29.1%, P=0.083). Using the same comparative data, a forest plot is provided in Figure 8. These results complement the statistical analysis of Table 9 and suggest that changes in pain scores between the two groups were essentially equivalent under either fixed or random effects assumptions.
Full table

Full table
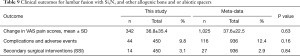
Full table
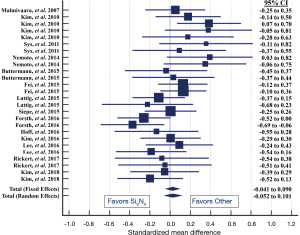
Table 9 also shows that the complications or adverse events and SSIs were statistically equivalent. The complication rate of the Si3N4 patients was ~9.8% compared to ~12.4% for the metadata (P=0.16). There were 14 SSI incidents for the Si3N4 patients and 27 for patients included in the meta-analysis (P=0.84). Additional details on complications, adverse events, and SSI are provided in Table 10. A recurrence of symptoms was the most common complication within the Si3N4 group (n=23, 5.1%), followed by a diagnosis of ALD (n=11, 2.4%). Repeat surgeries were performed on 14 patients for ALD and pedicle screw problems. Patients from the metadata group had similar complications and SSIs.
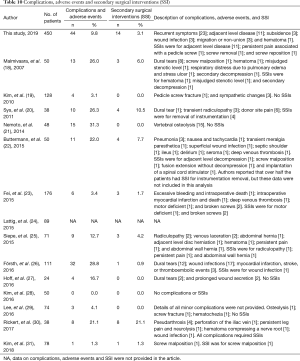
Full table
Discussion
Clinical effectiveness of Si3N4
Although Si3N4 has only recently emerged in the past decade as an effective bioceramic, it is well-known for its capabilities as an industrial material (38). Initially, its strength and toughness made it desirable as a structural biomaterial (39), but its enhanced osteoconductivity (40-42), bacteriostasis (43-46), improved radiolucency (47,48), lack of subsidence in the cervical spine (49), and wear resistance (50,51) are more relevant properties for spinal fusion and arthroplasty. In preclinical studies, Si3N4’s unique surface chemistry, topography, and hydrophilicity upregulate osteogenic activity to achieve faster spinal fusion, while simultaneously preventing bacterial adhesion and biofilm formation (52-57). As an orthopaedic articulation material, Si3N4 exhibits antioxidative characteristics which protect and potentially lengthen the service life of polyethylene liners (58). In contrast to oxide-based bioceramics which are purported to be bioinert and used solely for structural purposes (59), Si3N4 combines both bioactivity and structural stability in one material (60).
Although a number of recent clinical publications have reported on the performance of Si3N4 in cervical fusion (61-63), there are few contemporary reports on lumbar outcomes. Yet, Si3N4 uniquely has the longest clinical history as a spinal arthrodesis material. It was first used in a 30-patient lumbar spinal fusion study that was initiated in the mid-1980s (10). A 30+ years, follow-up of the remaining patients from this study was recently published (11). It showed that VAS pain scores and fusion effectiveness were similar to outcomes from the present study. Initial reductions of up to 47 points in VAS pain were seen during the first 5 years post-operatively with lower reductions after about 10 years (i.e., 35 points). Complication rates were slightly higher (n=11, 36.7%, P<0.01), but this is not unexpected given the design of these early devices. More recent case reports by Youssef (48) and Rambo (64) have demonstrated that Si3N4 was also effective in achieving solid lumbar fusion at 1-year follow-up in two patients, and it aided in the remediation of two other patients who had septic lumbar discitis, respectively. There is also an ongoing prospective randomized controlled lumbar fusion study comparing Si3N4 to PEEK devices that is expected to be published in the near future (65).
Minimum clinical important differences in pain scores
The present study is the largest multicenter evaluation of the safety and efficacy of Si3N4 for lumbar fusion to date. It demonstrates that Si3N4 cages implanted using various surgical approaches by different surgeons are as effective as other lumbar fusion implants and procedures from the compiled metadata of 1,025 patients in 26 cohorts and 14 published studies. The average reduction in VAS pain scores (ΔVAS) with the Si3N4 cages was 36.8±35.4 points compared to the average for the metadata of 37.6±22.5 points (P=0.63). The results from the present study also compare favorably with similar data from a recent systematic review of lumbar fusion by Phillips et al. (14). They reported that ΔVAS back pain scores for 3,060 patients compiled from 26 studies were 36.8±14.7 points (P=1.00 when compared to Si3N4 patients of this study, or P=0.19 when compared to the metadata of this study). This large compilation provides further evidence that clinical outcomes using Si3N4 cages are equivalent to other commonly used spacers or cages.
A number of studies or critical reviews have also attempted to quantify the minimum clinically important difference (MCID) for reductions in VAS back and leg pain for patients undergoing lumbar spine fusion (66-72). MCID represents “smallest change reported by patients that correlates with the patient stating that he or she is moderately better” (73), but the assessment methodology remains open to debate because there exists a considerable range in MCID values (0 to 100-point scale). As examples, Copay et al. suggested that a change of greater than 12 points for back pain and 16-points for leg pain were appropriate MCIDs for data retrospectively extracted from 454 lumbar fusion cases using a variety of surgical approaches (67). Hägg et al. recommended an 18–19-point reduction as the MCID for back pain based on the clinical evaluation of 289 lumbar cases performed using four different non-operative and surgical procedures including conservative management, instrumented and non-instrumented posterior lateral fusion (PLF), and instrumented PLIF (66). Parker proposed that the MCID values be set at 21 points for back pain and 28 points for leg pain based on 45 TLIF cases (69). Carragee et al. selected a 30-point decrease in pain intensity as the MCID regardless of its origin for 165 consecutive lumbar patients diagnosed with either isthmic spondylolisthesis or degenerative disc disease (68). The MCID values recommended by Solberg for 894 patients diagnosed with herniated discs were 25 for back pain and 35 for leg-pain (71). Finally, Zannikos reviewed these and other prior studies and concluded that agreeing on specific MCID values remains an important measure but requires further study and quantification (72). However, of note, the mean change in VAS scores for the Si3N4 patients of the present study exceeded all of the above cited or recommended MCID values. The effectiveness of the Si3N4 implants from this study can also be determined by the proportion of patients exceeding preset MCID targets. Based on the review given above, MCIDs of either 10-, 20-, 30-, or even 40-point result in 77.5%, 71.6%, 59.7%, and 51.8%, of the Si3N4 patients with successful outcomes, respectively. Clearly, a majority of the patients in the present study had noticable clinical improvements which were on par with the other large cohort studies cited previously.
Limitations
The retrospective design of the present study is a limitation. While data were compiled from the medical charts of patients by an independent examiner using a prescribed protocol, the original recording of this information was performed by the respective medical staffs of four surgical centers without a common procedure. Consequently, the use of a consistent set of clinical evaluation methods was lacking, including standards for reporting and recording VAS pain scores. Furthermore, differentiation between back, leg, and bodily pain was not monitored. However, mitigating this limitation is the fact that numerical pain rating scales of zero to 10 (or zero to 100) are easy to administer and evaluate. An additional limitation is the lack of consistent follow-up periods between the four centers. The study was also limited by a lack of contemporaneous controls. No data were acquired on any other cage materials at the four clinical sites. Only the compiled metadata and cited systematic and other reviews in the discussion section were used as comparative controls. Lastly, although the data were acquired by an unbiased medical records contractor, subsequent analyses were done by the study authors who are users of Si3N4 spinal spacers. Nonetheless, the statistical analyses and comparison to previously-published metadata and reviews fairly represent expected outcomes by other practitioners and surgical centers.
Conclusions
This study reports on the multicenter clinical outcomes of 450 patients who were implanted with Si3N4 intervertebral spacers/cages using various lumbar spinal fusion procedures. Patient follow-up averaged 11.4±9.8 months. Pre-op demographics, comorbidities, and VAS pain scores were compiled along with last follow-up pain scores, complications, adverse events, and SSIs. As a control group, comparative metadata were collected from 26 publications comprising 14 cohorts and 1,025 patients. The results demonstrated that implanted Si3N4 devices were safe and provided equivalent pain reduction outcomes to other commonly used spacers or cages implanted under differing surgical approaches. Although the four centers in this study were heterogeneous in pre-op patient demographics, comorbidities, and pre- and post-op clinical outcomes, the compiled ordinal and nominal data for the Si3N4 patients were statistically equivalent to the selected metadata. Also, the ΔVAS outcomes from this study were also equivalent to the results from a recent comprehensive systematic review. Lastly, a comparative MCID pain analyses demonstrated that Si3N4 cages were as effective in achieving the same level pain reduction as other lumbar arthrodesis devices or procedures.
Acknowledgments
Donald W. Guthner of Orgenix, LLC is acknowledged for the collection and initial analysis of patient data from the four surgical centers. Appreciation is expressed for assistance from medical, office, and administrative personnel at each of the surgical centers. Biomedical Statistical Consulting is acknowledged for their review and recommendations in performing the meta-analysis.
Financial Disclosure: The study was designed and funded by Amedica Corporation (now SINTX Technologies), Salt Lake City, UT USA, of which Dr. Bryan J. McEntire and Dr. B. Sonny Bal are officers and employees.
Footnote
Conflicts of Interest: Drs. GC Calvert, G VanBuren Huffmon III, WM Rambo Jr, and MW Smith were consulting surgeons to Amedica Corporation during the course of this study. Dr. BJ McEntire and Dr. BS Bal were principals and employees of Amedica Corporation (now SINTX Technologies).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Due to the retrospective nature of the study, informed consent by the respective IRBs of each surgical center was not required. However, none of the patients’ personal data was disclosed, and their records remain fully secured and in compliance with IRB standards.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baliga S, Treon K, Craig NJA. Low Back Pain: Current Surgical Approaches. Asian Spine J 2015;9:645-57. [Crossref] [PubMed]
- Cohen SP, Argoff CE, Carragee EJ. The Management of Low Back Pain. BMJ 2008;337:a2718. [Crossref] [PubMed]
- DePalma MJ, Ketchum JM, Saullo T. What Is the Source of Chronic Low Back Pain and Does Age Play a Role? Pain Med 2011;12:224-33. [Crossref] [PubMed]
- Taher F, Essig D, Lebl DR, et al. Lumbar Degenerative Disc Disease: Current and Future Concepts of Diagnosis and Management. Adv Orthop 2012;2012:970752.
- Katchko K, Schneider AD, Hsu WK. Lumbar Interbody Fusion Implant Materials. Contemp Spine Surg 2017;18:1-8. [Crossref]
- Landham PR, Don AS, Robertson PA. Do Position and Size Matter? An Analysis of Cage and Placement Variables for Optimum Lordosis in PLIF Reconstruction. Eur Spine J 2017;26:2843-50. [Crossref] [PubMed]
- Phan K, Mobbs RJ. Evolution of Design of Interbody Cages for Anterior Lumbar Interbody Fusion. Orthop Surg 2016;8:270-7. [Crossref] [PubMed]
- Kurtz SM. Development and Clinical Performance of PEEK Intervertebral Cages 2nd ed. PEEK Biomaterials Handbook. Elsevier Inc., 2019:263-80.
- Rao PJ, Pelletier MH. Spine Interbody Implants: Material Selection and Modification, Functionalization and Bioactivation of Surfaces to Improve Osseointegration. Orthop Surg 2014;6:81-9. [Crossref] [PubMed]
- Sorrell CC, Hardcastle PH, Druitt RK, et al. Results of 15-Year Clinical Study of Reaction Bonded Silicon Nitride Intervertebral Spacers. Proc 7th World Biomater Conf 2004;1872.
- Mobbs RJ, Rao PJ, Phan K, et al. Anterior Lumbar Interbody Fusion Using Reaction Bonded Silicon Nitride Implants: Long Term Case Series of the First Synthetic ALIF Spacer Implanted in Humans. World Neurosurg 2018;120:256-64. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Malham G, et al. Lumbar Interbody Fusion: Techniques, Indications and Comparison of Interbody Fusion Options Including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2-18. [PubMed]
- Mayer HM. The ALIF Concept. Eur Spine J 2000;9:S35-43. [Crossref] [PubMed]
- Phillips FM, Slosar PJ, Youssef JA, et al. Lumbar Spine Fusion for Chronic Low Back Pain Due to Degenerative Disc Disease. Spine J 2012;12:S147-8. [Crossref]
- Bydon M, De la Garza-Ramos R, Macki M, et al. Lumbar Fusion Versus Nonoperative Management for Treatment of Discogenic Low Back Pain. J Spinal Disord Tech 2014;27:297-304. [Crossref] [PubMed]
- Phan K, Thayaparan GK, Mobbs RJ. Anterior Lumbar Interbody Fusion versus Transforaminal Lumbar Interbody Fusion - Systematic Review and Meta-Analysis. Br J Neurosurg 2015;29:705-11. [Crossref] [PubMed]
- Yavin D, Casha S, Wiebe S, et al. Lumbar Fusion for Degenerative Disease: A Systematic Review and Meta-Analysis. Neurosurgery 2017;80:701-15. [Crossref] [PubMed]
- Malmivaara A, Slatis P, Heliovaara M, et al. Surgical or Nonoperative Treatment for Lumbar Spinal Stenosis? A Randomized Controlled Trial. Spine (Phila Pa 1976) 2007;32:1-8. [Crossref] [PubMed]
- Kim JS, Lee KY, Lee SH, et al. Which Lumbar Interbody Fusion Technique is Better in Terms of Level for the Treatment of Unstable Isthmic Spondylolisthesis? J Neurosurg Spine 2010;12:171-7. [Crossref] [PubMed]
- Sys J, Weyler J, Van Der Zijden T, et al. Platelet-Rich Plasma in Mono-Segmental Posterior Lumbar Interbody Fusion. Eur Spine J 2011;20:1650-7. [Crossref] [PubMed]
- Nemoto O, Asazuma T, Yato Y, et al. Comparison of Fusion Rates following Transforaminal Lumbar Interbody Fusion using Polyetheretherketone Cages or Titanium Cages with Transpedicular Instrumentation. Eur Spine J 2014;23:2150-5. [Crossref] [PubMed]
- Buttermann GR, Mullin WJ. Two-Level Circumferential Lumbar Fusion Comparing Midline and Paraspinal Posterior Approach. J Spinal Disord Tech 2015;28:E534-43. [Crossref] [PubMed]
- Fei H, Xu J, Wang S, et al. Comparison between Posterior Dynamic Stabilization and Posterior Lumbar Interbody Fusion in the Treatment of Degenerative Disc Disease: A Prospective Cohort Study. J Orthop Surg Res 2015;10:87. [Crossref] [PubMed]
- Lattig F, Fekete TF, Kleinstuck FS, et al. Lumbar Facet Joint Effusion on MRI as a Sign of Unstable Degenerative Spondylolisthesis: Should It Influence the Treatment Decision? J Spinal Disord Tech 2015;28:95-100. [Crossref] [PubMed]
- Siepe CJ, Stosch-Wiechert K, Heider F, et al. Anterior Stand-Alone Fusion Revisited: A Prospective Clinical, X-ray and CT Investigation. Eur Spine J 2015;24:838-51. [Crossref] [PubMed]
- Försth P, Ólafsson G, Carlsson T, et al. A Randomized, Controlled Trial of Fusion Surgery for Lumbar Spinal Stenosis. N Engl J Med 2016;374:1413-23. [Crossref] [PubMed]
- Hoff EK, Strube P, Pumberger M, et al. ALIF and Total Disc Replacement versus 2-Level Circumferential Fusion with TLIF: A Prospective, Randomized, Clinical and Radiological Trial. Eur Spine J 2016;25:1558-66. [Crossref] [PubMed]
- Kim CW, Doerr TM, Luna IY, et al. Minimally Invasive Transforaminal Lumbar Interbody Fusion Using Expandable Technology: A Clinical and Radiographic Analysis of 50 Patients. World Neurosurg 2016;90:228-35. [Crossref] [PubMed]
- Lee JH, Kong CB, Yang JJ, et al. Comparison of Fusion Rate and Clinical Results Between CaO-SiO2-P2O5-B2O3 Bioactive Glass Ceramics Spacer with Titanium Cages in Posterior Lumbar Interbody Fusion. Spine J 2016;16:1367-76. [Crossref] [PubMed]
- Rickert M, Fleege C, Tarhan T, et al. Transforaminal Lumbar Interbody Fusion Using Polyetheretherketone Oblique Cages with and without a Titanium Coating. Bone Joint J 2017;99-B:1366-72. [Crossref] [PubMed]
- Kim HJ, Kang KT, Chun HJ, et al. Comparative Study of 1-year Clinical and Radiological Outcomes Using Robot-Assisted Pedicle Screw Fixation and Freehand Technique in Posterior Lumbar Interbody Fusion: A Prospective, Randomized Controlled Trial. Int J Med Robot 2018;14:e1917. [Crossref] [PubMed]
- Moher D, Shamseer L, Clarke M, et al. Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) 2015 Statement. Syst Rev 2015;4:1. [Crossref] [PubMed]
- Thomas BN. Are You Heavier or Shorter than the Average American? CNN. December 20, 2018 (accessed 08/15/19).
- Wang TW, Asman K, Gentzke AS, et al. Tobacco Product Use Among Adults -United States, 2017. MMWR Morb Mortal Wkly Rep 2018;67:1225-32. [Crossref] [PubMed]
- Go AS, Mozaffarian D, Roger VL, et al. Heart Disease and Stroke Statistics—2013 Update. Circulation 2013;127.
- National Diabetes Statistics Report, 2017. US Department of Health and Human Services. National Center for Chronic Disease Prevention and Health Promotion 2017.
- Wright NC, Looker AC, Saag KG, et al. The Recent Prevalence of Osteoporosis and Low Bone Mass in the United States Based on Bone Mineral Density at the Femoral Neck or Lumbar Spine. J Bone Miner Res 2014;29:2520-6. [Crossref] [PubMed]
- Riley FL. Silicon Nitride and Related Materials. J Am Ceram Soc 2000;83:245-65. [Crossref]
- Bal BS, Rahaman MN. Orthopedic Applications of Silicon Nitride Ceramics. Acta Biomater 2012;8:2889-98. [Crossref] [PubMed]
- Pezzotti G, Marin E, Adachi T, et al. Bioactive Silicon Nitride: A New Therapeutic Material for Osteoarthropathy. Sci Rep 2017;7:44848. [Crossref] [PubMed]
- Pezzotti G, McEntire BJ, Bock RM, et al. In Situ Spectroscopic Screening of Osteosarcoma Living Cells on Stoichiometry-Modulated Silicon Nitride Bioceramic Surfaces. ACS Biomater Sci Eng 2016;2:1121-34. [Crossref]
- Pezzotti G, McEntire BJ, Bock R, et al. Silicon Nitride: A Synthetic Mineral for Vertebrate Biology. Sci Rep 2016;6:31717. [Crossref] [PubMed]
- Webster TJ, Patel AA, Rahaman MN, et al. Anti-Infective and Osteointegration Properties of Silicon Nitride, Poly (Ether Ether Ketone), and Titanium Implants. Acta Biomater 2012;8:4447-54. [Crossref] [PubMed]
- Gorth DJ, Puckett S, Ercan B, et al. Decreased Bacteria Activity on Si3N4 Surfaces Compared with PEEK or Titanium. Int J Nanomedicine 2012;7:4829-40. [PubMed]
- Bock RM, Jones EN, Ray DA, et al. Bacteriostatic Behavior of Surface-Modulated Silicon Nitride in Comparison to Polyetheretherketone and Titanium. J Biomed Mater Res A 2017;105:1521-34. [Crossref] [PubMed]
- Pezzotti G. A Spontaneous Solid-State NO Donor to Fight Antibiotic Resistant Bacteria. Mater Today Chem 2018;9:80-90. [Crossref]
- Anderson M, Bernero J, Brodke D. Medical Imaging Characteristics of Silicon Nitride Ceramic A New Material for Spinal Arthroplasty Implants. In: 8th Annual Spine Arthroplasty Society Global Symposium on Motion Preservation Technology. Miami, FL; 2008:547.
- Youssef JA, Myhre SL, Bal BS. Radiographic Follow-up of Transforaminal Lumbar Fusion with Silicon Nitride Spacers: A Case Report of Two Patients. J Musculoskelet Disord Treat 2016;2:1-8. [Crossref]
- Suh PB, Puttlitz C, Lewis C, et al. The Effect of Cervical Interbody Cage Morphology, Material Composition, and Bone Density on Subsidence Risk. J Am Acad Orthop Surg 2016.160-8.
- McEntire BJ, Lakshminarayanan R, Ray DA, et al. Silicon Nitride Bearings for Total Joint Arthroplasty. Lubricants 2016;4:1-24. [Crossref]
- McEntire BJ, Puppulin L, Bal BS, et al. Data-Driven Innovations in Joint Replacement: Do Ceramic Femoral Heads Contribute to Polyethylene Oxidation? J Ceram Sci Technol 2017;7:1-6.
- Bock RM, McEntire BJ, Bal BS, et al. Surface Modulation of Silicon Nitride Ceramics for Orthopaedic Applications. Acta Biomater 2015;26:318-30. [Crossref] [PubMed]
- Bock RM, Marin E, Rondinella A, et al. Development of a SiYAlON Glaze for Improved Osteoconductivity of Implantable Medical Devices. J Biomed Mater Res B Appl Biomater 2018;106:1084-96. [Crossref] [PubMed]
- Pezzotti G, Oba N, Zhu W, et al. Human Osteoblasts Grow Transitional Si/N Apatite in Quickly Osteointegrated Si3N4 Cervical Insert. Acta Biomater 2017;64:411-20. [Crossref] [PubMed]
- Pezzotti G, Bock RM, McEntire BJ, et al. In vitro Antibacterial Activity of Oxide and Non-Oxide Bioceramics for Arthroplastic Devices: I. In situ Time-Lapse Raman Spectroscopy. Analyst 2018;143:3708-21. [Crossref] [PubMed]
- Boschetto F, Toyama N, Horiguchi S, et al. In vitro Antibacterial Activity of Oxide and Non-Oxide Bioceramics for Arthroplastic Devices: II. In situ Time-Lapse Fourier Transform Infrared Spectroscopy. Analyst 2018;143:2128-40. [Crossref] [PubMed]
- Boschetto F, Fainozzi D, Marin E, et al. Monitoring Metabolic Reactions in Staphylococcus epidermidis Exposed to Silicon Nitride using in situ Time-Lapse Raman Spectroscopy. J Biomed Opt 2018;23:1-10. [Crossref] [PubMed]
- Pezzotti G, Zhu W, Sugano N, et al. Oxide Ceramic Femoral Heads Contribute to the Oxidation of Polyethylene Liners in Artificial Hip Joints. J Mech Behav Biomed Mater 2018;82:168-82. [Crossref] [PubMed]
- Pezzotti G. Bioceramics are not Bioinert. Mater Today 2017;20:395-8. [Crossref]
- Pezzotti G. Silicon Nitride: A Bioceramic with a Gift. ACS Appl Mater Interfaces 2019;acsami.9b07997.
- Ball HT, McEntire BJ, Bal BS. Accelerated Cervical Fusion of Silicon Nitride versus PEEK Spacers: A Comparative Clinical Study. J Spine 2017;6:1000396. [Crossref]
- Smith MW, Romano DR, McEntire BJ, et al. A Single Center Retrospective Clinical Evaluation of Anterior Cervical Discectomy and Fusion Comparing Allograft Spacers to Silicon Nitride Cages. J Spine Surg 2018;4:349-60. [Crossref] [PubMed]
- Arts MP, Wolfs JFC, Corbin TP. Porous Silicon Nitride Spacers versus PEEK Cages for Anterior Cervical Discectomy and Fusion: Clinical and Radiological Results of a Single-Blinded Randomized Controlled Trial. Eur Spine J 2017;26:2372-9. [Crossref] [PubMed]
- Rambo WM. Treatment of Lumbar Discitis using Silicon Nitride Spinal Spacers: A Case Series and Literature Review. Int J Surg Case Rep 2018;43:61-8. [Crossref] [PubMed]
- Kersten RF, van Gaalen SM, Arts MP, et al. The SNAP Trial: A Double Blind Multi-Center Randomized Controlled Trial of a Silicon Nitride Versus a PEEK Cage in Transforaminal Lumbar Interbody Fusion in Patients with Symptomatic Degenerative Lumbar Disc Disorders: Study Protocol. BMC Musculoskelet Disord 2014;15:57. [Crossref] [PubMed]
- Hägg O, Fritzell P, Nordwall A. The Clinical Importance of Changes in Outcome Scores After Treatment for Chronic Low Back Pain. Eur Spine J 2003;12:12-20. [Crossref] [PubMed]
- Copay AG, Glassman SD, Subach BR, et al. Minimum Clinically Important Difference in Lumbar Spine Surgery Patients: A Choice of Methods Using the Oswestry Disability Index, Medical Outcomes Study Questionnaire Short Form 36, and Pain Scales. Spine J 2008;8:968-74. [Crossref] [PubMed]
- Carragee EJ, Cheng I. Minimum Acceptable Outcomes after Lumbar Spinal Fusion. Spine J 2010;10:313-20. [Crossref] [PubMed]
- Parker SL, Adogwa O, Paul AR, et al. Utility of Minimum Clinically Important Difference in Assessing Pain, Disability, and Health State after Transforaminal Lumbar Interbody Fusion for Degenerative Lumbar Spondylolisthesis. J Neurosurg Spine 2011;14:598-604. [Crossref] [PubMed]
- Djurasovic M, Glassman SD, Dimar JR, et al. Does Fusion Status Correlate with Patient Outcomes in Lumbar Spinal Fusion? Spine (Phila Pa 1976) 2011;36:404-9. [Crossref] [PubMed]
- Solberg T, Johnsen LG, Nygaard ØP, et al. Can We Define Success Criteria for Lumbar Disc Surgery? Estimates for a Substantial Amount of Improvement in Core Outcome Measures. Acta Orthop 2013;84:196-201. [Crossref] [PubMed]
- Zannikos S, Lee L, Smith HE. Minimum Clinically Important Difference and Substantial Clinical Benefit: Does One Size Fit All Diagnoses and Patients? Semin Spine Surg 2014;26:8-11. [Crossref]
- Farrar JT, Portenoy RK, Berlin JA, et al. Defining the Clinically Important Difference in Pain Outcome Measures. Pain 2000;88:287-94. [Crossref] [PubMed]