Tantalum: the next biomaterial in spine surgery?
Introduction
Tantalum is a biocompatible, relatively inert transition metal whose first reported use was as a component of surgical sutures by Burke in 1940 (1-5). Since its introduction, it has successfully been used in various orthopedic fields, dentistry, hernia repair, vascular anastomoses, neural reconstruction and cranio-facial fields (3,6). Porous tantalum is an open-cell structure composed of tantalum in a repeating dodecahedron pattern creating an appearance similar to cancellous bone (6). Scaffolds of porous tantalum have been manufactured to have a small elastic modulus (3–25 GPa) (7-11). Porous tantalum’s elastic modulus is similar to that of cancellous bone (3.78 GPa) and cortical bone (14.64 GPa), thus reducing shielding, while having a ten-fold higher bend strength (6,12,13). Tantalum has also been shown to have relatively high frictional characteristics, which allows it to maintain a strong initial stability against bone compared to other materials (14,15). A sign of the high biocompatibility of tantalum is seen by its excellent corrosion resistance (16). This resistance is provided by a stable oxide (Ta2O5) coating the surface of the metal which is occasionally accompanied by macrophages, but is associated with little to no inflammatory reaction (17,18).
One of the most appealing aspects of porous tantalum is its high porosity, with 75–80% of the material’s volume composed of pores (19). Moreover, the porosity of tantalum is higher than other common metals used in orthopedics such as Regenerex (67%), Titanium (60%), CoCr beads (30–50%), fiber metal (40–50%) and CSTi (50–60%). Porous implants, through their ability to allow bone, vascular and other tissue infiltration, are effective in providing stability for implants secondary to biological fixation. Sagomonyants et al. showed that human osteoblasts from elderly (>60 years old) female patients grew at a significantly higher rate on tantalum substrate compared with titanium fiber mesh and tissue culture plastic, with 4–6 and 12–16 times greater growth, respectively (20). A more recent study by Wang et al. showed that osteoblasts cultured on porous tantalum samples adhered to the surface and pore walls by day 3. By week 12 the surface and pores were fully covered by interwoven bone, demonstrating that tantalum is an ideal material for adhesion and proliferation of osteoblasts as well as infiltration of nutrients (21).
Additionally, animal studies using porous tantalum have been promising. A 2019 study of osteointegration of porous tantalum in the lumbar spine of rabbits found equivalent radiographic fusion scores compared to iliac crest autograft at 12 months post-operatively (22). Another study of porous tantalum in the cervical spine in a goat model showed that the percentage of bone at the implant margins at 6 weeks was 35%. Bony ingrowth was approximately twice as high as polyetheretherketone (PEEK) implants at all time points tested (23). A lumbar spine study done in pigs using porous tantalum showed that at 3 months post-operation 11% of tissue growth was from bone, 6% from bone marrow and the rest from fibrous tissue (24).
Anecdotal reports of osteointegration of porous tantalum in human subjects has been reported in acetabular shells, femoral stems, tibial trays and patella specimens, showing variable levels of bone ingrowth (25). One report, included in this review, of ingrowth in a cervical spine porous tantalum specimen ex-planted 7 months post-operatively showed primarily lamellar bone surrounding vascular channels in approximately 50% of the pores, with 83% of the bone having been formed de novo and no inflammatory response (26). Additionally, porous tantalum implants have a low rate of infection. A study done by Yang et al. saw the use of tantalum for treatment of spinal infections found significant improvements in pain and functional scores at 1-year follow-up, with a 0% re-infection rate (27). In fact, of the studies included in this review, a total of 4 patients (1.3%) out of 316 patients treated with tantalum products experienced infections.
These biologic properties of tantalum make it a favorable metal to utilize in spinal fusion surgery. Clinical studies published on the use of tantalum for this purpose have been promising. As of this time, there have been only a handful of studies incorporating porous tantalum in spinal surgery, most of which have been cohort studies with low enrollment. Since 2015, the published literature on clinical outcomes of tantalum in spine surgery has more than doubled, yet there have been no reviews synthesizing the findings of these novel studies. The objective of the current review is to describe these studies in order to characterize and summarize the clinical and radiographic outcomes associated with the use of tantalum metal in spine fusion surgery.
Materials and methods
A review of the literature was performed on the PubMed (MEDLINE) database on January 27, 2019, for papers pertinent to the use of tantalum metal in spine surgery. A multitude of search terms were used including: “tantalum”, “screw”, “instrumentation”, “interbody”, “fusion”, “spine”, and “spine surgery”. Relevant articles were reviewed using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement as a guideline. Two researchers individually screened all full title abstracts and confirmed the eligibility of each paper. A third reviewer was used to address any conflicts.
Studies were reviewed for eligibility based on determined inclusion and exclusion criteria. Study inclusion criteria included English written published randomized control trials, and prospective and retrospective cohort studies that included the use of tantalum metal in spine surgery in human subjects for any etiology. Exclusion criteria included studies that used animals, that were published prior to 2000, that used tantalum outside of the spine, with less than ten patients, and that used tantalum with other metals.
Through our analysis of the studies included in this review, the authors compared studies based on the surgical procedure selected, number of patients included in the study, age and sex of the patients, spinal anatomy operated on, time to last follow-up, and type of imaging used. Primary outcomes examined in the present study include: pain and functional scores, operative time, blood loss, hospital stay, complications, subsidence, adjacent segment degeneration (ASD), fragmentation, cobb angle, fusion rate, and revision rate. Studies were subcategorized into tantalum usage in the lumbar and cervical spine for outcome comparison.
Statistical analysis was performed separately on cervical and lumbar studies. Fusion rates, revision rates and complications were assessed with weighted means based on number of patients treated with that specific modality. Data was not sufficient enough for statistical analysis of lumbar studies. Independent students t-tests were performed with all possible pairings. Independent samples t-tests were also performed comparing treatment with fusion rate, revision rate, and complication rate at final follow-up as reported by each study and compared with the other modalities. All analyses were performed on SPSS version 25 on Windows PC. P values less than 0.05 were considered significant.
Results
Search results
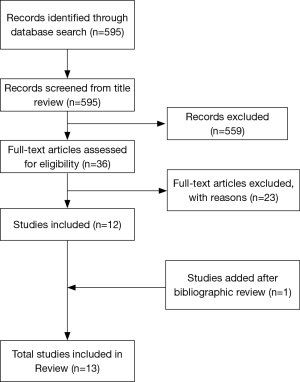
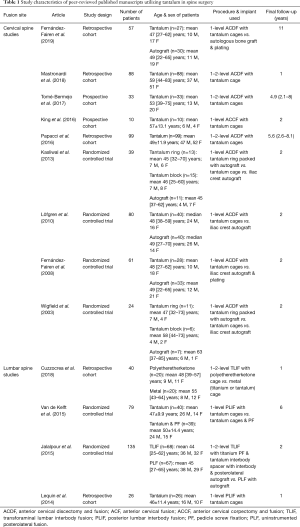
Our initial search yielded 595 entries, which was subsequently reduced down to 13 papers after title, abstract and full-text review. Further bibliography review yielded one additional paper for a total of 14 studies based on our predetermined inclusion and exclusion criteria (Figure 1). One study (Yang et al.) was not used in this review due to their use of Tantalum for spinal infections, which was noted in the introduction. The studies were then grouped based on their use of tantalum on the lumbar or cervical spine. Our search yielded four studies on lumbar fusion and ten studies on cervical fusion (Table 1).
Full table
Fusion rates
Evaluating fusion rates for patients with cervical treatment (follow-up ranged from 1 to 11 years), we found increased fusion rates in autograft alone when compared to those treated with tantalum standalone without graft supplementation (92.8% vs. 89.0%, P=0.001) and those treated with tantalum cages plus autograft (92.8% vs. 64.8%, P<0.0001). Tantalum standalone fusion rates were higher when compared to those treated with tantalum ring with autograft (89.0% vs. 64.8%, P=0.002), while the tantalum standalone fusion rates were less when compared to those treated with autograft with anterior plate (89.0% vs. 92.0%, P=0.031). In the setting of lumbar fusion (follow-up ranged from 6 months to 6.8 years), patients treated with autograft had lower fusion rate as compared to tantalum standalone (80.0% vs. 93.4%, P<0.0001). The number of studies for lumbar fusion were scant and thus making it difficult to make conclusions about lumbar fusion rates compared to cervical fusion rates. There was insufficient data for statistical comparisons of lumbar fusion rates using other treatment methods.
Complication rates
Complication rates in cervical fusion were lower in patients treated with tantalum standalone versus those treated with autograft (7.4% vs. 13.7%, P<0.0001), and autograft and anterior plate (7.4% vs. 33%, P=0.001). Tantalum ring with autograft patients had a lower complication rate than those treated with only autograft (7.7% vs. 13.7%, P<0.001), and no significant difference in complication rate when compared to those treated with tantalum standalone only (7.7% vs. 7.3%, P=0.423). There was insufficient data for statistical comparisons of lumbar complication rates.
Revision surgery rates
Tantalum standalone patients had a lower rate of revision surgery when compared to those treated with autograft (2.8% vs. 12.8%, P<0.0001) and those treated with autograft and anterior plate (2.8% vs. 8.0%, P<0.001), but higher rate when compared to those treated with tantalum ring and autograft (2.8% vs. 0.0%, P<0.0001); note that only one study had presented revision data for tantalum ring with autograft. There was insufficient data for statistical comparisons of lumbar revision rates.
Discussion
Porous tantalum use in lumbar spinal fusion surgery
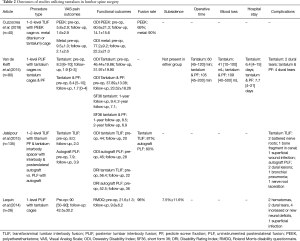
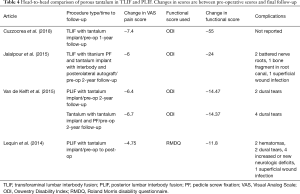
Our search of the literature yielded four studies that utilized tantalum in spinal fusion surgery involving the lumbar spine (Table 2) (28-31). The studies applied two different types of surgical approaches for fusion with tantalum: Cuzzocrea et al. and Jalalpour et al. studied transforaminal lumbar interbody fusion (TLIF), while Van de Kelft et al. and Lequin et al. studied posterior lumbar interbody fusion (PLIF).
Full table
Interbody cages have been designed using a multitude of biomaterials, with titanium and PEEK showing a high degree of success in achieving arthrodesis. However, in one study, higher elastic modulus of titanium resulted in poorer clinical outcomes compared to morselized bone (28). Cuzzocrea et al., retrospectively studied the use of TLIF in 40 patients treated with either PEEK or interbody metal, either titanium or tantalum, with no metal selection criteria specified. At 1-year follow-up they found similar, significant improvements in pain and functional scores in both groups compared to pre-operative scores (Table 2). Fusion rates were higher in the metal group compared to the PEEK group at 90% of patients achieving fusion vs. 69% achieving fusion, respectively. Cuzzocrea et al. attributed this to the superior osteointegrative properties of metal cages and lower incidence of peri-prosthetic osteolysis that they found in their patients, leading to more stable interbody fusion. The study did not differentiate results between titanium and tantalum and thus conclusions on tantalum use in TLIF cannot be inferred from this paper, but the study provides strong evidence for the use of interbody cages made from metal over PEEK.
The randomized controlled trial (RCT) done by Jalalpour et al. compares the use of tantalum interbody spacer in TLIF with pedicle screw fixation vs. posterolateral fusion (PLF) with autograft for patients suffering from more than 1 year of low back pain. Although patients were assigned to treatment groups randomly, the TLIF group had 41% of their patients operated at two levels, while the PLF group had 24% of patients have two-level fusion. Additionally, PLF patients had more patients undergo L5–S1 fusion compared to the TLIF group (42% vs. 25%). They found a statistically significant difference in improvement of Visual Analog Scale (VAS) pain score, and Disability Rating Index (DRI) and global assessment functional scores between their two groups, at 2-year follow-up, in favor of the tantalum TLIF group. Additionally, their PLF group saw an increased revision rate compared to the TLIF tantalum groups (17.9% vs. 7.4%) due to segmental degeneration, local pain, bone graft fragmentation vs. pseudarthrosis, respectively. This study found a fusion rate similar to that achieved by Cuzzocrea et al. in their titanium/tantalum metal group, with 87% rate in the tantalum group.
Two older studies, Van de Kelft et al. and Lequin et al., looked at the use of tantalum in PLIF procedure. Van de Kelft had two groups in their study with a follow-up of around 6 years for both: one treated with PLIF using tantalum stand-alone and another treated with PLIF using tantalum and a pedicle screw fixation. At 24-month follow-up, there were no significant differences in pain and functional scores in the two study groups, but significantly less operative time and blood loss in the group that did not have the additional pedicle fixation, as they expected. Moreover, patients in the pedicle screw fixation group saw more dural tears (10% vs. 5%), a higher revision rate (2.5% vs. 0%) due to improper screw placement, but less ASD (2.6% vs. 9%), although not statistically significant. With similar clinical and radiographic results at long-term follow-up of 6 years in the group, the authors in this study concluded that tantalum can effectively be used in the PLIF procedure without pedicle screw fixation (Table 2).
Lequin et al. performed a retrospective study using tantalum stand-alone cages in PLIF. They saw improvements in pain (VAS) and functional (Roland Morris Disability Questionnaire) scores at 15.3-month follow-up, with good subjective outcomes; yet they were unable to calculate significance due to insufficient pre-operative scores. They saw a complication rate of 34.6%, with a 15% revision rate secondary to hematoma formation, cerebrospinal fluid (CSF) leak, recurrent severe back pain or translucency around cage. This complication and revision rates were higher than those found in the Van de Kelft study, which specified that a single surgeon performed all the operations. Lequin did have a 96% fusion rate at 15.3-month follow-up. This was the highest fusion rate in the literature for tantalum use in lumbar spine, which could be possibly due to the longer follow-up allowing more time for osseous integration. The study yielded a mean subsidence of 7.5%±11.6% (similar to that described in the literature), but also found disc height significantly increased at 1-year follow-up (P<0.001) (Table 2). This rate of subsidence was higher than that found in the Van de Kelft study, even though both studies measured subsidence the same way. The authors note that their subsidence percentage correlates with what is described in the literature. They argue that in the short-term, subsidence is not correlated with clinical outcomes (31), nor does additional posterior pedicle screw fixation improve rates of subsidence (32,33).
Assessing differences in outcomes between TLIF and PLIF is difficult due to a lack of a head-to-head studies We found similar improvements in VAS back pain scores in the four studies at their last follow-up (Table 3). The PLIF studies were also more likely to cause dural tears, while the TLIF studies had higher risks of nerve injuries.
Full table
Porous tantalum use in cervical spinal fusion surgery
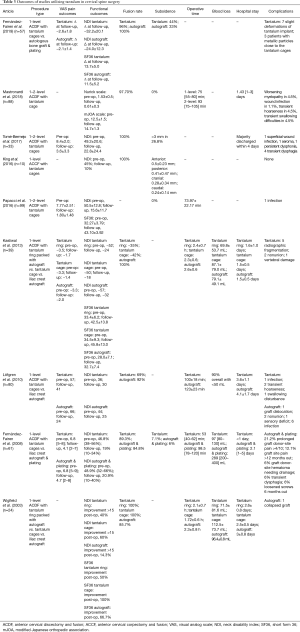
Nine manuscripts were identified for their use of tantalum metal in cervical spine fusion, ranging from 2003 to 2018 (Table 4). Eight of the nine studies described the use of tantalum in anterior cervical discectomy and fusion (ACDF), while one of the studies, King et al., utilized anterior cervical corpectomy and fusion (ACCF).
Full table
The King et al. study retrospectively looked at ten patients treated with ACCF using tantalum stand-alone cages (34). They found stable cervical lordosis and 100% fusion rates at 2-year follow-up. Rates of subsidence appeared to decrease over time, and all patients experienced improved clinical outcomes, with zero patients undergoing revision surgery (Table 4). Their data supports the use of tantalum stand-alone cages in up to two-level ACCF.
ACDF using autograft from iliac bone graft has been in use since the 1950s (35,36). The clinical results are typically satisfactory, but infections, hematomas and longstanding pain from donor site are frequently described in the literature. Allografts come with risks of producing an immunogenic response from the host, disturbing fusion healing (37). Thus, the search for an implant that could model bone has been sought after for ACDF. Porous tantalum has been found to be more osteoconductive than other available biomaterials as mentioned previously (6,7,19).
Three comparison studies were identified examining tantalum versus autograft. The two Fernandez-Fairen studies differed in length of follow-up, 11- vs. 2-year follow-up (38,39). The Lofgren study differed in that it did not use plating with its autograft like the two Fernandez-Fairen studies did, and thus used autograft as a stand-alone construct (37). All three studies found similar improvements in functional and pain scores between their two groups, with the 2018 Fernandez-Fairen study showing that these improvements last at least 11 years, the longest follow-up time known to date. The studies found significantly less operative time (P<0.05) and blood loss (P<0.05) for their tantalum groups compared to the autograft groups, but similar days of hospitalization (Table 4).
As noted earlier, the morbidity of autograft at donor site is of significant concern. The 2018 Fernandez-Fairen study found 21.1% of their patients suffering from 4/10 pain at donor site, with 12.1% have this pain for >12 months. Additionally, 6% of patients suffered from hematoma formation at donor site, 6% had loosened screws and 3% had loosened plates in the autograft group. Lofgren et al. only reported one complication at 2-year follow-up of sensory deficit in their autograft group (Table 4). These complications can be avoided with the use of porous tantalum, decreasing the morbidity of spinal fusion.
Rates of subsidence were higher in the tantalum groups for both Fernandez-Fairen studies. The 2018 study saw 44% of patients treated with tantalum have a mean of 2.2-mm subsidence, while their autograft group saw 33% of patients afflicted with a mean of 0.5-mm subsidence, with no progression after the 6-week time point. This subsidence was not significantly correlated with associated lordosis or kyphosis of any segment in any group (P>0.1). Additionally, with similar outcomes in both groups, this subsidence does not appear to be clinically or radiographically correlated at 11-year follow-up. In their 2008 study, which was at a much shorter follow-up, tantalum group was 7.1% of patients afflicted with 3-mm subsidence, and in their autograft group, 6% were affected with 1–2-mm subsidence. The subsidence rate found in the 2018 paper was consistent with the range found with other cages such as titanium, carbon or PEEK (Table 4) (40-42). The 2018 study also notes the higher rate of post-operative kyphotic alignment in the tantalum group, which they attribute to the fact that the tantalum stand-alone group did not use a plate (43,44)). Additionally, their rate of ASD was similar between groups, and similar to reported ASD in ACDF reported in the literature. The onset of ASD after fusion using tantalum did not correlate with the postoperative sagittal alignment nor the subsidence of the implant (P>0.5). In fact, Fernandez-Fairen, notes that the ASD might not lead to significant clinical deterioration. They found that radiographic ASD was not correlated with clinical deterioration; only the ODOM score was correlated with radiographic deterioration. None of the patients in this study that suffered from ASD had to have revision surgery and were managed successfully by conservative treatment.
Fusion rates were similar in both the Fernandez-Fairen studies between the tantalum and autograft groups (2018: 96% vs. 100%, 2008: 89.3% vs. 84.4%, respectively, neither was significantly different). It is interesting to note that in the Fernandez-Fairen study from 2018 that had an 11-year follow-up, both groups had higher fusion rates, suggesting osseous integration, especially for tantalum, may take 2 or more years. The Lofgren et al. study found a significant difference in fusion rates at 2-year follow-up with the tantalum group showing 69% fusion and the autograft group showing 92% fusion (Table 4). They attribute this low fusion rate to a couple of factors. First, although they used the same criteria for evaluating fusion radiographically, they had more stringent criteria for measuring movement between spinal processes (45). Second, they did not remove smokers from the analysis as the Fernandez-Fairen study did. In light of this data, however, all three studies reported lower revision rates for non-union in their tantalum groups. Mastronardi et al. also noted differences in fusion rates for smokers and non-smokers in their tantalum treated ACDF group; they saw a fusion rate of 87.8% vs. 48.9% after 6 months in their non-smokers vs. smokers, respectively. Although, both of these groups reach 100% at 12 months (46).
Three studies looked at tantalum stand-alone in ACDF treatment that further provide strong support for the use of tantalum in cervical spine fusion. Tomé-Bermejo et al., Mastronardi et al. and Papacci et al., all found significant improvements in measured pain and functional scores at last follow-up (46-48). Operative time in Matronardi et al. and Papacci et al. and hospitalization times in Mastronardi et al. and Tomé-Bermejo et al. were similar to other studies in this review that used tantalum. In terms of subsidence, Mastronardi et al. and Papacci et al. reported 0% subsidence rate, while Tomé-Bermejo et al. saw 26.82% subsidence in 11 discs (Table 4). They note that during the process of bone remodeling, it is expected that cages will settle <2 mm into the vertebral body until fusion occurs, whereas if more than that occurs, it may be secondary to inappropriate, intraoperative endplate preparation. They found subsidence lead to no subjective and clinical difference in their patients, consistent with Fernadez-Fairen found in both their studies.
Mastronardi et al., Tomé-Bermejo et al. and Papacci et al. had 0%, 0%, and 1% ASD, while both Mastronardi and Tomé-Bermejo had 0% fragmentation rate in their tantalum treated patients. Fusion rates in these two studies were 97.7% and 100%, and only Mastronardi and Papacci had to revise operations (2.3% of patients for implant failure) and 1.0% revision rate for infected hardware, respectively.
Two studies looked at tantalum stand-alone cages in ACDF placement in addition to autograft control and tantalum ring (filled with iliac crest autograft) groups: Kasliwal et al. and Wigfield et al. (26,49). Both studies had 2-year follow-up and found improvements in pain and functional scores in all of their groups; more so in the tantalum groups, but not statistically significant. It is important to note that the Wigfield study used an older tantalum construct (Hedrocel in the form of Novus ring or block). Both studies had similar operative times, blood loss and number of days hospitalized in all three arms of their studies and were comparable to other studies included in this review (Table 4). Wigfield saw a 0% fragmentation rate, while Kasliwal saw fragmentation in 27.8% of their tantalum patients, which they attributed to failure to fusion in these patients at 24 months. Additionally, Wigfield had a 0% revision rate for tantalum groups and 14.28% revision for control, while Kasliwal had a 5.12% revision rate for tantalum groups secondary to non-union.
The most significant result from the Kasliwal study is their low fusion rate. Whereas, they found 100% fusion in their autograft group, their tantalum groups were only 38% fused at 2-year follow-up. The authors do not provide any explanation for their poor fusion rates (Table 4). This sharply contrasts what was found in the Wigfield study, which had to halt study recruitment when they saw low fusion rates in the tantalum groups initially, where they found 100% fusion rate at 24-month follow-up for both tantalum groups and an 85.7% fusion rate for their control group. The authors state their concern of having a large rate of revision surgery due to low fusion rates as a reason to halt the study, even though clinically the patients were not affected. They attribute this late fusion to two factors. First, a study done by AO ASIF Research Institute that observed bone remodeling initially might be a temporary porosis associated with necrosis due to periosteal damage or interruption of blood supply. Once the blood supply is restored, bony ingrowth can proceed (50). Their second reason is due to difficulties in radiographic interpretation of fusion. They state that the high radiopacity of tantalum makes it easily visible on radiographs but makes it difficult to assess the bridging trabecular bone for assessment of fusion. Thus, angulation with dynamic images becomes more important for this purpose (49). They determined fusion by looking at angulation (<2 degrees) and alterations of interspinous process distance (<2 mm), which are more stringent criteria than other studies in this review. A study done by Levi et al. found that tantalum produced more streak artifact on CT, but less on MRI, permitting MRI reading to be better able to image surrounding bony structures to assess fusion (51). Blumenthal et al. suggested that plain radiographs might underestimate the degree of fusion in 1 out of 5 cases, with different thresholds of accepted angulation leading to vastly different rates of fusion (52). Both studies state that fusion rates were not correlated with clinical outcomes.
Conclusions
Tantalum application has demonstrated extraordinary benefit in several subspecialties, including hip arthroplasty revision and has a potential role in spine surgery. Its use in the lumbar spine has demonstrated excellent fusion rates and outcome scores comparable to those of other interbody cages in the literature. PLIF and TLIF studies suggest a potentially higher rate of subsidence with tantalum use however no apparent clinical consequence. In case series, low rates of subsidence and acceptable clinical outcomes are noted in up to 2 level ACDF surgery. Fusion rates in short-term studies evaluating tantalum in the cervical spine are conflicting, although long-term series beyond 2 years show excellent fusion rates. This early finding is partially related to the difficulty in radiographic evaluation of fusion in the setting of tantalum cage use. Further studies are needed to tease out the timing of fusion with the implementation of tantalum in the cervical spine. Blood loss, operative time, and hospital length of stay are more likely related to approach and harvesting of autograft than individualized cage composition.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Burke GL. The corrosion of metals in tissues; and an introduction to tantalum. Can Med Assoc J 1940;43:125-8. [PubMed]
- Matsuno H, Yokoyama A, Watari F, et al. Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium. Biomaterials 2001;22:1253-62. [Crossref] [PubMed]
- Mohandas G, Oskolkov N, McMahon MT, et al. Porous tantalum and tantalum oxide nanoparticles for regenerative medicine. Acta Neurobiol Exp (Wars) 2014;74:188-96. [PubMed]
- Niinomi M, Nakai M, Hieda J. Development of new metallic alloys for biomedical applications. Acta Biomater 2012;8:3888-903. [Crossref] [PubMed]
- Okazaki Y, Gotoh E. Comparison of metal release from various metallic biomaterials in vitro. Biomaterials 2005;26:11-21. [Crossref] [PubMed]
- Levine BR, Sporer S, Poggie RA, et al. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials 2006;27:4671-81. [Crossref] [PubMed]
- Cohen R. A porous tantalum trabecular metal: basic science. Am J Orthop (Belle Mead NJ) 2002;31:216-7. [PubMed]
- Liu H, Lin J, Roy K. Effect of 3D scaffold and dynamic culture condition on the global gene expression profile of mouse embryonic stem cells. Biomaterials 2006;27:5978-89. [Crossref] [PubMed]
- Mikhael MM, Huddleston PM, Zobitz ME, et al. Mechanical strength of bone allografts subjected to chemical sterilization and other terminal processing methods. J Biomech 2008;41:2816-20. [Crossref] [PubMed]
- Stemper BD, Board D, Yoganandan N, et al. Biomechanical properties of human thoracic spine disc segments. J Craniovertebr Junction Spine 2010;1:18-22. [Crossref] [PubMed]
- Welldon KJ, Atkins GJ, Howie DW, et al. Primary human osteoblasts grow into porous tantalum and maintain an osteoblastic phenotype. J Biomed Mater Res A 2008;84:691-701. [Crossref] [PubMed]
- Heary RF, Parvathreddy N, Sampath S, et al. Elastic modulus in the selection of interbody implants. J Spine Surg 2017;3:163-7. [Crossref] [PubMed]
- Zardiackas LD, Parsell DE, Dillon LD, et al. Structure, metallurgy, and mechanical properties of a porous tantalum foam. J Biomed Mater Res 2001;58:180-7. [Crossref] [PubMed]
- Levine B. A New Era in Porous Metals: Applications in Orthopaedics. Adv Eng Mater 2008;10:788-92. [Crossref]
- Zhang Y, Ahn P, Fitzpatrick D, et al. Interfacial frictional behavior: cancellous bone, cortical bone, and a novel porous tantalum biomaterial. J Musculoskelet Res 1999;3:245-51. [Crossref]
- Tahal D, Madhavan K, Chieng LO, et al. Metals in Spine. World Neurosurg 2017;100:619-27. [Crossref] [PubMed]
- Bobyn JD, Toh KK, Hacking SA, et al. Tissue response to porous tantalum acetabular cups: a canine model. J Arthroplasty 1999;14:347-54. [Crossref] [PubMed]
- Sidhu KS, Prochnow TD, Schmitt P, et al. Anterior cervical interbody fusion with rhBMP-2 and tantalum in a goat model. Spine J 2001;1:331-40. [Crossref] [PubMed]
- Bobyn JD, Stackpool GJ, Hacking SA, et al. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J Bone Joint Surg Br 1999;81:907-14. [Crossref] [PubMed]
- Sagomonyants KB, Hakim-Zargar M, Jhaveri A, et al. Porous tantalum stimulates the proliferation and osteogenesis of osteoblasts from elderly female patients. J Orthop Res 2011;29:609-16. [Crossref] [PubMed]
- Wang Q, Zhang H, Li Q, et al. Biocompatibility and osteogenic properties of porous tantalum. Exp Ther Med 2015;9:780-6. [Crossref] [PubMed]
- Lu M, Xu S, Lei ZX, et al. Application of a novel porous tantalum implant in rabbit anterior lumbar spine fusion model: in vitro and in vivo experiments. Chin Med J (Engl) 2019;132:51-62. [Crossref] [PubMed]
- Sinclair SK, Konz GJ, Dawson JM, et al. Host bone response to polyetheretherketone versus porous tantalum implants for cervical spinal fusion in a goat model. Spine 2012;37:E571-80. [Crossref] [PubMed]
- Zou X, Li H, Bunger M, et al. Bone ingrowth characteristics of porous tantalum and carbon fiber interbody devices: an experimental study in pigs. Spine J 2004;4:99-105. [Crossref] [PubMed]
- Hanzlik JA, Day JS. Acknowledged Contributors: Ingrowth Retrieval Study G. Bone ingrowth in well-fixed retrieved porous tantalum implants. J Arthroplasty 2013;28:922-7. [Crossref] [PubMed]
- Wigfield C, Robertson J, Gill S, et al. Clinical experience with porous tantalum cervical interbody implants in a prospective randomized controlled trial. Br J Neurosurg 2003;17:418-25. [Crossref] [PubMed]
- Yang SC, Chen HS, Kao YH, et al. Single-stage anterior debridement and reconstruction with tantalum mesh cage for complicated infectious spondylitis. World J Orthop 2017;8:710-8. [Crossref] [PubMed]
- Cuzzocrea F, Ivone A, Jannelli E, et al. PEEK versus metal cages in posterior lumbar interbody fusion: a clinical and radiological comparative study. Musculoskelet Surg 2019;103:237-41. [Crossref] [PubMed]
- Jalalpour K, Neumann P, Johansson C, et al. A Randomized Controlled Trial Comparing Transforaminal Lumbar Interbody Fusion and Uninstrumented Posterolateral Fusion in the Degenerative Lumbar Spine. Global Spine J 2015;5:322-8. [Crossref] [PubMed]
- Lequin MB, Verbaan D, Bouma GJ. Posterior lumbar interbody fusion with stand-alone Trabecular Metal cages for repeatedly recurrent lumbar disc herniation and back pain. J Neurosurg Spine 2014;20:617-22. [Crossref] [PubMed]
- Van de Kelft E, Van Goethem J. Trabecular metal spacers as standalone or with pedicle screw augmentation, in posterior lumbar interbody fusion: a prospective, randomized controlled trial. Eur Spine J 2015;24:2597-606. [Crossref] [PubMed]
- Marchi L, Abdala N, Oliveira L, et al. Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. J Neurosurg Spine 2013;19:110-8. [Crossref] [PubMed]
- Tokuhashi Y, Ajiro Y, Umezawa N. Subsidence of metal interbody cage after posterior lumbar interbody fusion with pedicle screw fixation. Orthopedics 2009;32. [PubMed]
- King V, Swart A, Winder MJ. Tantalum trabecular metal implants in anterior cervical corpectomy and fusion: 2-year prospective analysis. J Clin Neurosci 2016;32:91-4. [Crossref] [PubMed]
- Cloward RB. The treatment of ruptured lumbar intervertebral discs by vertebral body fusion. I. Indications, operative technique, after care. J Neurosurg 1953;10:154-68. [Crossref] [PubMed]
- Smith GW, Robinson RA. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am 1958;40-A:607-24. [Crossref] [PubMed]
- Löfgren H, Engquist M, Hoffmann P, et al. Clinical and radiological evaluation of Trabecular Metal and the Smith-Robinson technique in anterior cervical fusion for degenerative disease: a prospective, randomized, controlled study with 2-year follow-up. Eur Spine J 2010;19:464-73. [Crossref] [PubMed]
- Fernández-Fairen M, Alvarado E, Torres A. Eleven-Year Follow-Up of Two Cohorts of Patients Comparing Stand-Alone Porous Tantalum Cage Versus Autologous Bone Graft and Plating in Anterior Cervical Fusions. World Neurosurg 2019;122:e156-67. [Crossref] [PubMed]
- Fernández-Fairen M, Sala P, Dufoo M Jr, et al. Anterior cervical fusion with tantalum implant: a prospective randomized controlled study. Spine (Phila Pa 1976) 2008;33:465-72. [Crossref] [PubMed]
- Kast E, Derakhshani S, Bothmann M, et al. Subsidence after anterior cervical inter-body fusion. A randomized prospective clinical trial. Neurosurg Rev 2009;32:207-14; discussion 214. [Crossref] [PubMed]
- Pinder EM, Sharp DJ. Cage subsidence after anterior cervical discectomy and fusion using a cage alone or combined with anterior plate fixation. J Orthop Surg (Hong Kong) 2016;24:97-100. [Crossref] [PubMed]
- Zajonz D, Franke AC, von der Hoh N, et al. Is the radiographic subsidence of stand-alone cages associated with adverse clinical outcomes after cervical spine fusion? An observational cohort study with 2-year follow-up outcome scoring. Patient Saf Surg 2014;8:43. [PubMed]
- Troyanovich SJ, Stroink AR, Kattner KA, et al. Does anterior plating maintain cervical lordosis versus conventional fusion techniques? A retrospective analysis of patients receiving single-level fusions. J Spinal Disord Tech 2002;15:69-74. [Crossref] [PubMed]
- Yue WM, Brodner W, Highland TR. Long-term results after anterior cervical discectomy and fusion with allograft and plating: a 5- to 11-year radiologic and clinical follow-up study. Spine (Phila Pa 1976) 2005;30:2138-44. [Crossref] [PubMed]
- Fassett DR, Apfelbaum RI, Hipp JA. Comparison of fusion assessment techniques: computer-assisted versus manual measurements. J Neurosurg Spine 2008;8:544-7. [Crossref] [PubMed]
- Mastronardi L, Roperto R, Cacciotti G, et al. Anterior Cervical Fusion with Stand-alone Trabecular Metal Cages to Treat Cervical Myelopathy Caused by Degenerative Disk Disease. Observations in 88 Cases with Minimum 12-month Follow-up. J Neurol Surg A Cent Eur Neurosurg 2018;79:496-501. [Crossref] [PubMed]
- Papacci F, Rigante L, Fernandez E, et al. Anterior cervical discectomy and interbody fusion with porous tantalum implant. Results in a series with long-term follow-up. J Clin Neurosci 2016;33:159-62. [Crossref] [PubMed]
- Tomé-Bermejo F, Morales-Valencia JA, Moreno-Pérez J, et al. Degenerative Cervical Disc Disease: Long-term Changes in Sagittal Alignment and Their Clinical Implications After Cervical Interbody Fusion Cage Subsidence: A Prospective Study With Standalone Lordotic Tantalum Cages. Clin Spine Surg 2017;30:E648-55. [Crossref] [PubMed]
- Kasliwal MK, Baskin DS, Traynelis VC. Failure of porous tantalum cervical interbody fusion devices: two-year results from a prospective, randomized, multicenter clinical study. J Spinal Disord Tech 2013;26:239-45. [Crossref] [PubMed]
- Lim TH, Kwon H, Jeon CH, et al. Effect of endplate conditions and bone mineral density on the compressive strength of the graft-endplate interface in anterior cervical spine fusion. Spine (Phila Pa 1976) 2001;26:951-6. [Crossref] [PubMed]
- Levi AD, Choi WG, Keller PJ, et al. The radiographic and imaging characteristics of porous tantalum implants within the human cervical spine. Spine (Phila Pa 1976) 1998;23:1245-50; discussion 1251. [Crossref] [PubMed]
- Blumenthal SL, Gill K. Can lumbar spine radiographs accurately determine fusion in postoperative patients? Correlation of routine radiographs with a second surgical look at lumbar fusions. Spine (Phila Pa 1976) 1993;18:1186-9. [Crossref] [PubMed]