M6-C cervical disc replacement failure associated with late onset infection
Introduction
Anterior cervical discectomy and fusion (ACDF) has been considered the gold standard for the treatment of degenerative cervical disc disease (1). Recently, cervical disc arthroplasty (CDA) has been developed as an alternative to fusion as they aim to maintain the natural biomechanics of the spine, reducing the risk of adjacent segment degeneration, a known side effect of spinal fusions (1,2). We present the first reported case of a CDA failure in an M6-C cervical disc (Spinal Kinetics, Sunnyvale, CA, USA), associated with an infection presenting three years post-implantation.
Case presentation
Presentation
A 59-year-old male, who was a keen swimmer and surfer, presented with a six-week history of neck pain and left upper limb pain in a C7 distribution, without associated sensory disturbance. Clinical examination revealed positive Spurling sign with subtle weakness of the left triceps and pectoralis with a depressed triceps jerk. MRI cervical spine showed a large left C6/7 paracentral and foraminal disc protrusion with impingement of the C7 nerve root.
Management
The patient was initially managed non-operatively, with oral analgesia supplemented by a left C7 peri-radicular injection. Although temporarily effective, the patient developed worsening radicular pain and left upper limb weakness, prompting the decision to proceed with surgery via a C6/7 disc replacement.
Operation
A routine anterior cervical approach was utilised. A C6/7 discectomy was performed and the posterior longitudinal ligament divided to achieve satisfactory decompression of the thecal sac and C7 nerve roots. Following endplate preparation, a 7×17×14 mm3 M6-C disc replacement was inserted with intraoperative imaging being utilised to ensure appropriate implant positioning.
Post-operative course
Following surgery, the patient reported immediate symptom resolution and had an uncomplicated hospital admission. Follow-up with serial imaging was undertaken at 3, 6 and 12 months post-operation, at which time the patient remained asymptomatic.
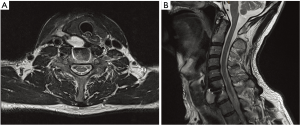
Three years post-operatively, the patient presented with one month of dysphagia associated with a palpable neck mass. There were no associated neck or radicular pain or systemic features of infection. CT and MRI cervical spine identified a contrast enhancing right prevertebral collection adjacent to the prosthesis (Figure 1). Routine blood tests were unremarkable, with normal white cell count and C-reactive protein. An imaging-guided fine needle aspirate was undertaken for culture, with no organisms identified. Progress imaging showed reaccumulating of this collection and the decision was made to proceed with surgical exploration.
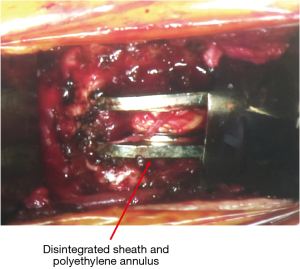
A standard anterior cervical approach via the previous incision was performed revealing encapsulated necrotic debris. This extended to the disc replacement and appeared contiguous. On inspection of the implant, there appeared to be obvious failure with disintegration of the sheath and polyethylene artificial annulus and exposure of the artificial nucleus (Figure 2). The residual prosthesis was removed and the surgical field aggressively debrided and washed. A 9 mm, 9-degree lordotic cage with cadaveric bone was inserted into the disc space. Empirical antibiotics were started following the operation.
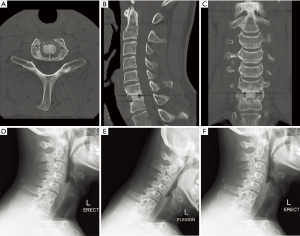
Post-operative course was unremarkable with improvement of his dysphagia. Multiple intraoperative swabs grew Propionibacterium acnes (P. acnes) sensitive to penicillins and moxifloxacin. Intravenous benzylpenicillin and moxifloxacin was continued for six weeks with ongoing amoxicillin. Serial imaging showed no recurrence of the collection and solid bony union (Figure 3).
Discussion
CDA surgery has become more popular in its use for degenerative disc disease as an alternative to the gold standard of an ACDF (1). The main benefit of a disc replacement is to provide a device that mimics the natural movements of a native disc preserving the normal biomechanics of the spine and thus avoiding adjacent segment disease (2). The M6-C cervical disc replacement is a novel device composed of a polycarbonate urethane polymeric core surrounded by a polyethylene woven fibre construct that is specifically designed to replicate the anatomical, physiological and biomechanical characteristics of a native disc (3).
Common complications relating to the approach to CDA were similar to that of ACDF (4). These include superficial or deep infections, dysphagia, wound haematoma, epidural haematoma, oesophageal or laryngeal injuries, recurrent laryngeal palsy, dural laceration, cerebrospinal fluid leak, Horner’s syndrome, vertebral artery, carotid artery or jugular vein injury and thoracic duct injury (4). Dysphagia and cervical wound infections are the most common with rates reported up to 27.2% and 22.5% respectively (5).
Cervical infections discussed in the literature on common complications of CDA frequently present as superficial skin infection and commonly early in the post- operative period (5). Overall, anterior cervical spine approach operations have low infection rates given the use of blunt dissection in avascular plans avoiding direct muscular tissue trauma and the extensive lymphatic drainage in the region (6). Our case is unusual due to its delayed presentation. Literature suggests late infections are typically associated with oesophageal perforations, implant migration, Zenker’s diverticulum and bacterial seeding from another surgical site or bacteremia (7).
Christiano et al. describes a case with a patient who presents with delayed dysphagia with raised white cell count and C-reactive protein and a deep peri-prosthesis abscess two years following an ACDF (8). The patient underwent surgical exploration and Streptococcus intermedius, a commensal of the mouth and upper respiratory tract (9), was cultured. In our case, P. acnes was isolated suggesting a difference source of infection.
It has been hypothesized over the last 15 years that subclinical, chronic P. acnes, a common skin commensal, infection within the intervertebral disc space causes disc degeneration and the accompanying chronic lower back pain (10). Animal models have shown a direct correlation of P. acnes and disc degeneration (10). Ganko et al. published a systematic review and meta-analysis of data correlating P. acnes to degenerative disc disease (11). They concluded that the pooled prevalence of disc infection in 602 patients was 36.2% (11) and P. acnes was found in the majority of the disc infections accounting for 59.6% (11). Capoor et al. also looked at the correlation of P. acnes with disc degeneration (10). They found bacterial biofilm detected in situ via confocal scanning laser microscopy and this was present within the body of the herniated disc, not on the surface (which would be expected in cases of contamination) (10). P. acnes biofilm were also detected by fluorescence in situ hybridization (10). This microscopic evidence of a P. acnes biofilm supports the notion that there may be an association between chronic disc infection and disc degeneration.
Implant failure following the M6-C cervical disc replacement is infrequently reported. Gradual collapse of the endplate and subsidence has been described in two patients as well as prosthesis loosening in a patient involved in a motor vehicle accident four months post-operation (1). Brenke et al. reported a case of the M6-C artificial disc replacement prosthesis which had herniated its core contents causing spinal cord compression and myelopathy (12). No distinctive patient features such as increased mechanical stress was identified and the authors speculated the scenario of fatigue to the surrounding sheath leading to the breakdown (12).
In our case two hypotheses exists. The first is that P. acnes was present in the native disc at the time of surgery and an indolent infection produced a prosthesis biofilm leading to destruction of the surrounding sheath and mechanical failure of the prosthesis. The other is that fatigue of the surrounding sheath and mechanical failure of the device occurred first and the fragmented parts led to the infection.
In cases of anterior disc replacements with associated infection, the literature supports explantation of such device followed by a fusion (4). A corpectomy may be necessary if too much bone is removed during the procedure (4). For patients with malalignment or subsidence in the absence of neurological symptoms, another option would be to leave the disc intact and perform a posterior fusion (13). The choice of bone substitute for cervical fusions are variable. A comparison of fusion rates of allograft and autologous bone graft showed no significant difference in fusion rates for one and two level anterior cervical fusions, although the allograft group did take longer for fusion to occur (14).
Conclusions
CDA is recognized as a good alternative to ACDF. We present an interesting case of a patient with a delayed presentation of dysphagia leading to the discovery of a concurrent P. acnes infection and implant failure. Revision surgery with an anterior fusion and long-term antibiotics resulted in resolution of his symptoms and treatment of his infection.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Thomas S, Willems K, Van den Daelen L, et al. The M6-C Cervical Disk Prosthesis: First Clinical Experience in 33 Patients. Clin Spine Surg 2016;29:E182-7. [Crossref] [PubMed]
- Park JB, Chang H, Yeom JS, et al. Revision surgeries following artificial disc replacement of cervical spine. Acta Orthop Traumatol Turc 2016;50:610-8. [Crossref] [PubMed]
- Lauryssen C, Coric D, Dimmig T, et al. Cervical total disc replacement using a novel compressible prosthesis: Results from a prospective Food and Drug Administration-regulated feasibility study with 24-month follow-up. Int J Spine Surg 2012;6:71-7. [Crossref] [PubMed]
- Skovrlj B, Lee DH, Caridi JM, et al. Reoperations Following Cervical Disc Replacement. Asian Spine J 2015;9:471-82. [Crossref] [PubMed]
- Xu JC, Goel C, Shriver MF, et al. Adverse Events Following Cervical Disc Arthroplasty: A Systematic Review. Global Spine J 2018;8:178-89. [Crossref] [PubMed]
- De la Garza-Ramos R, Abt NB, Kerezoudis P, et al. Deep-wound and organ-space infection after surgery for degenerative spine disease: an analysis from 2006 to 2012. Neurol Res 2016;38:117-23. [Crossref] [PubMed]
- Jin SW, Kim SH, Choi JI, et al. Late infection from anterior cervical discectomy and fusion after twenty years. Korean J Spine 2014;11:22-4. [Crossref] [PubMed]
- Christiano LD, Goldstein IM. Late prevertebral abscess after anterior cervical fusion. Spine (Phila Pa 1976) 2011;36:E798-802. [Crossref] [PubMed]
- Ramhmdani S, Bydon A. Streptococcus intermedius: an unusual cause of spinal epidural abscess. J Spine Surg 2017;3:243-9. [Crossref] [PubMed]
- Capoor MN, Ruzicka F, Schmitz JE, et al. Propionibacterium acnes biofilm is present in intervertebral discs of patients undergoing microdiscectomy. PLoS One. 2017;12:e0174518. [Crossref] [PubMed]
- Ganko R, Rao PJ, Phan K, et al. Can bacterial infection by low virulent organisms be a plausible cause for symptomatic disc degeneration? A systematic review. Spine (Phila Pa 1976) 2015;40:E587-92. [Crossref] [PubMed]
- Brenke C, Schmieder K, Barth M. Core herniation after implantation of a cervical artificial disc: case report. Eur Spine J 2015;24 Suppl 4:S536-9. [Crossref] [PubMed]
- Buchowski JM, Sekhon LHS, Yoon D, et al. Adverse events of cervical arthroplasty. Tech Orthop 2010;25:138-44. [Crossref]
- Kadam A, Millhouse PW, Kepler CK, et al. Bone substitutes and expanders in Spine Surgery: A review of their fusion efficacies. Int J Spine Surg 2016;10:33. [Crossref] [PubMed]