
Subsidence induced recurrent radiculopathy after staged two-level standalone endoscopic lumbar interbody fusion with a threaded cylindrical cage: a case report
Introduction
Expandable interbody fusion cage technology has become commercially available several years ago (1). Most of these systems are intended to be used with posterior supplemental fixation (2). The attraction for the endoscopic spine surgeon, who is performing his decompression surgeries in an outpatient ambulatory surgery center (ASC), is with standalone interbody fusion technology (3).This more simplified version of lumbar decompression fusion surgery may be the response to the call for more cost-effective ways to treat instability-induced lumbar spinal stenosis in the elderly who nowadays live longer and are functioning better in spite of medical comorbidities due to their advanced medical management (4). The feasibility of a standalone endoscopic interbody fusion with an expandable threaded cylindrical implant has been recently demonstrated (5) and validated in a two-year outcome study (4). The authors reported successful outcomes in the majority of patients. Only a small subset of patients required additional interventional or surgical aftercare mostly for subsidence-induced problems. Long-term follow-up problems with the procedure were hitherto unknown (4).
The focus of this case report is to call attention to additional problems with a standalone lumbar endoscopic decompression interbody fusion procedure using the VARILIF-L™ implant that may occur in the long-run after an initial uneventful postoperative course and how the authors elected to manage them. Therefore, we report on a 64-year-old female patient who underwent two separate staged outpatient standalone transforaminal endoscopic decompression fusion surgeries first at L4/5 for sciatica-type low back and leg pain due to spinal stenosis and Grade I spondylolisthesis and then at L5/S1 for failed conservative management of adjacent level disease which became symptomatic 11 months after the L4/5 endoscopic VARLIF™ surgery. After another six months following the L5/S1 VARLIF™, the patient sciatica returned due to recurrent compression of the L5 and S1 nerve roots from bulge of the remaining central portion of the annulus fibrosus and bony and scar tissue formation behind the cylindrical threaded expandable interbody fusion cage. This case report describes the clinical course and how the subsidence induced problems were managed with another endoscopic decompression procedure instead of open revision surgery.
Materials and methods
Patient selection criteria
Outpatient endoscopic spinal surgery programs for the treatment of lumbar herniated disc and spinal stenosis were established by the first author (KUL) In 2007 at the Center for Advanced Spine Care of Southern Arizona in Tucson Arizona (6). The senior author established his endoscopic outpatient spinal surgery program at the Squaw Peak Facility in Phoenix Arizona 21 years ago. It was later integrated into the Desert Institute of Spine Care founded by his son—Christopher Yeung—in 2003 (7). Patients treated with the transforaminal outside-in decompression procedure popularized by Hoogland et al. (8). The first author’s surgical techniques are a modification of the transforaminal approach initially described by Hoogland (8) and further popularized by Ruetten et al. who incorporated it into his full-endoscopic methodology (9). This platform was used to place a threaded interbody fusion device into the intervertebral space to treat instability-induced symptoms of degenerated disc disease that have proven refractory to non-operative treatment. The inclusion and exclusion criteria have been described in detail elsewhere (3). In brief, patients with lumbar radiculopathy, dysesthesias, and decreased motor function refractory to 12 weeks of conservative care due to Grade I spondylolisthesis with associated foraminal or lateral recess stenosis confirmed on magnetic resonance images (MRI), and computed tomography (CT) scans are appropriate candidates for the endoscopic standalone interbody fusion procedure. Patients with Grade II or higher spondylolisthesis, severe central stenosis (less than 100 mm2); (10), massive facet hypertrophy, infection, or metastatic disease are not suitable for this procedure. Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Surgical technique & implant
The interbody fusion procedure has been described in great detail (5). The initial step is a generous foraminoplasty to exposure the intervertebral disc space and the safe zone formed by the inferior pedicle and the exiting-, and traversing nerve roots. This may require a near-total removal of the superior articular process (SAP) via osteotomy from the distal pedicle to prepare the introitus for the cage insertion. For discectomy and endplate preparation, the working sheath may be advanced into the interspace using procedural steps of the “inside-out” (11) techniques. The patient was operated in the prone position under sedation and monitored anesthesia care. Endoscopic instruments required to complete the decompression and interspace preparation include motorized drills, Kerrison rongeurs, trephines most of which were deployed through the central 4.1 mm inner working channel of the spinal endoscope. Attention should be paid to align the instruments and the cage parallel to the endplates. Additional resection of the inferior articular process (IAP) or the superomedial pedicle wall may be required if there is severe loss of posterior disc height. The exiting nerve root should be carefully retracted to minimize irritation of its dorsal root ganglion (DRG) during cage insertion. Special cannulated instruments for these steps include paddle shavers and tapes which are also used to size the implant. During the decortication of the endplates, damage to the subchondral bone should be avoided to minimize implant subsidence. The first author and his team performed the surgeries. The second author assisted in the operations and the writing of this manuscript. The senior author has encountered the bony and scar tissue formation within the axillary hidden zone of Macnab countless times during the endoscopic revision of some of his over 11,000 transforaminal endoscopic decompression patients who experienced current radiculopathy after an asymptomatic interval following the index surgery. He added to the interpretation and contributed to the writing of this case report.
Clinical follow-up
After each surgery, the patient was typically evaluated at 2, 6, and 12 weeks, and after that at 6, and 12, postoperatively. Additional visits were scheduled to deal with any unexpected problems. Clinical improvements were evaluated by calculating reductions in the visual-analog scales (VAS) (12) for leg pain ranging from no pain [0] to worst pain [10] and the Oswestry Disability Index (ODI) (13). The VAS and ODI scores were correlated with any patient-related factors that could explain less favorable outcomes.
Case presentation
A 64-year-old female presented with a six-year history of progressively worsening right-sided sciatica-type leg and back pain with several acute on chronic episodes. On initial presentation, the patient’s imaging studies revealed Grade I spondylolisthesis at the L4/5 level with foraminal and lateral recess stenosis and unilateral radiculopathy. At the time of the surgical consultation with the first author, the patient had undergone extensive non-operative care with more than 6 months of failed physical therapy (PT), non-steroidal anti-inflammatories (NSAIDs), and three rounds of transforaminal epidural steroid injections (TESI) at the direction of her primary care physician (PCP). The first author performed a diagnostic work up linking her ongoing radiculopathy at the time of initial presentation to the stenotic process at the L4/5 level using diagnostic analgesic transforaminal epidural steroid injections containing 1% lidocaine. Decompression alone of the foraminal and lateral recess stenosis was deemed likely ineffective since the patient had Grade I spondylolisthesis at the L4/5 level. Although deemed rigid, the authors anticipated a vacuum disc and the need for an extensive foraminoplasty with near complete resection of the superior articular process (SAP)—both being prognosticators of less favorable outcomes with decompression alone. Therefore, the patient was consented for an L4/5 standalone transforaminal endoscopic decompression fusion surgery using the expandable threaded cylindrical VARILIF-L™ implant. The patient had a successful outcome from that surgery with near complete resolution of her preoperative symptoms reporting a VAS reduction of 6 for both leg and back pain and an overall improvement of function equivalent to a 51-point reduction on the ODI rating. Her walking endurance improved from 500 feet preoperatively to unlimited postoperatively. Only minimal implant subsidence was noted at the index level during the first 11 months of follow-up. At that time, the patient presented with new-onset of radicular pain which on the basis of history and physical examination, as well as an updated imaging- and diagnostic TESI work up was attributed to nerve root entrapment now symptomatic at the L5/S1 level. The patient was now treated for symptomatic adjacent level disease which limited her walking endurance to 1,000 feet. She underwent an additional three months of conservative care with physical therapy, NSAIDs, and TESI on the symptomatic right-side. Every analgesic TESI at the L5/S1 level produced a diagnostic short-term response within minutes of the injection without therapeutic pain relief. A preoperative computed tomography before the planned L5/S1 endoscopic standalone VARILIF™ fusion 15 months following her L4/5 VARILIF™ procedure revealed fusion at the L4/5 level with minimal subsidence of the VARILIF-L™ implant, and advanced degeneration of the L5/S1 motion segment with lateral recess and foraminal stenosis, reduced posterior disc height, and vacuum disc.
Consequently, the patient underwent uneventful L5/S1 endoscopic standalone fusion using the VARILIF-L™ implant with successful clinical outcome with a postoperative VAS reduction of 5 for both leg and back pain and an overall improvement of function equivalent to a 52-point reduction on the ODI rating with unlimited walking endurance within three months postoperatively. Six months after the endoscopic L5/S1 VARILIF™ procedure, she developed recurrent L5 and S1 radiculopathy on the same surgical approach side. Computed tomography showed significant implant subsidence and recurrence of a large soft tissue mass posterior to the interbody fusion cage on the approach side. Vertical and angular cage subsidence was noted preferentially on the side of the cage housing the expansion mechanism. This suggested that stress concentration induced vertical subsidence with loss of posterior disc height and the associated recurrence of bulge of the remaining medial annulus. Angular subsidence rotated the cage on the approach side into the axilla between the traversing S1 and exiting L5 nerve root causing compression (Figure 1). A brief six-weeks trial of non-operative care with activity modification, PT, NSAIDs, and TESI was unsuccessful. The patient requested surgical treatment of her unrelenting radiculopathy. After brief consideration of open revision surgery with wide compression shared decision with the patient was made for another transforaminal endoscopic decompression given her high satisfaction with the previous outpatient endoscopic surgeries (Figure 2). She underwent a generous transforaminal endoscopic foraminoplasty with resection of the remaining SAP and partial S1 pediculectomy. This allowed access to the compressive pathology. Direct intraoperative visualization during the decompression showed severe impingement of the traversing-S1 and exiting L5 nerve roots. There was a large central herniation, scar tissue tethered to both nerve roots and bone arising from the posterior ring apophysis in the hidden zone of Macnab. Presumably the surgical trauma from the cage insertion some 6 months prior and/or the subsidence of the standalone interbody fusion cage has caused the recurrent nerve root entrapment. The back of the VARILIF-L interbody fusion implant was exposed through the previous annulotomy site by resecting the annular scar tissue and freeing the L5 and S1 nerve roots by carefully dissecting them with an endoscopic elevator. The bone graft chamber of the VARILIF-L™ cage was found filled solidly with bone and the implant was found fused to both adjacent endplates upon exploration of spinal fusion. The patient was discharged from the ASC after an uneventful postoperative recovery reporting instant relief from her sciatica. At the time of the writing of this case report, she reported a VAS reduction of 5 for both leg and back pain and an overall improvement of function equivalent to a 57-point reduction on the ODI rating with unlimited walking endurance.
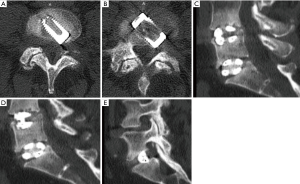
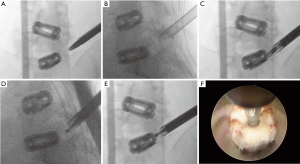
Discussion
In the mind of the experienced endoscopic spine surgeon, standalone transforaminal endoscopic decompression and fusion is the natural progression of the application of recent technology advancements in an outpatient ASC setting. While most expandable interbody fusion systems call for posterior supplemental pedicle screw fixation, the VARILIF-L™ system chosen by the authors does not require it and has been approved by the FDA for a standalone application (5). This team of authors has a great deal of experience with lumbar endoscopic decompression procedures and has developed highly reliable, published and validated clinical protocols that produce successful clinical outcomes with high patient satisfaction in the vast majority of patients (14-16) not just in the short-term but also in the long-run (11,17). This high patient satisfaction is primarily driven by the simplicity of the endoscopic surgery where patents may undergo complex decompression surgeries under local anesthesia and sedation through small incisions without having to be admitted to a hospital for complex medical care or postoperative pain control. Complication- and readmission rates are very low compared to traditional open and other forms of translaminar minimally invasive spinal surgeries (17-19). The standalone transforaminal endoscopic interbody fusion is within the same realm of outpatient surgeries that simply loses its attractiveness to patients when pedicle screws are involved. Patients are highly aware of pedicle screw induced problems due to hardware induced pain syndromes, and adjacent segment disease setting them seemingly up for repetitive surgery cycles. Likewise, surgeons and ASC operators are aware of higher blood loss, more extended surgery and fluoroscopy times, and higher intra- and postoperative complications ultimately increasing operational and implant costs negatively impacting reimbursement mainly if ASCs operate under bundled- or risk-sharing care models. Therefore, the need for simplified outpatient lumbar fusions is obvious.
The surgeons involved in the writing of this case were highly motivated to implement such a simplified outpatient lumbar decompression fusion programs suitable for ASC and have previously reported on the feasibility of standalone endoscopic decompression fusion using an expandable cylindrical threaded interbody fusion cage—the VARILIF-L™. The authors also reported on two-year clinical outcomes with the procedure and delineated patient inclusion/exclusion criteria and appropriate surgical indications which are likely to be associated with successful clinical outcomes in the majority of patients (3). The latter study also helped understand the risk factors for failed outcomes, some of which were caused by implant subsidence typically within the first five months from the index surgery. As with any new technology implementation, there is a learning curve, and clinical innovation may expose the limitations of such technology when unforeseen problems arise. Stenosis due to subsidence-induced loss of posterior disc height may ensue and cause the remaining annulus to bulge to a point where the patient developed recurrent symptoms. In addition, the surgical trauma from the index transforaminal cage insertion may have contributed to bone and scar tissue formation within the hidden zone of Macnab and contributed to painful tethering of the dorsal root ganglia of both the exiting L5 and traversing S1 nerve root. The senior author has routinely observed this phenomenon during some of his transforaminal endoscopic revision surgeries he performed on select patients with recurrent same level radiculopathy following prior transforaminal endoscopic decompression he encountered during his 28-year spanning career with over 11,000 endoscopic surgeries (Figure 3). As previously shown, the cylindrical implant frequently rotates and shows a combination of vertical and angular subsidence. In the case presented herein, the implant had turned and preferentially subsided into the inferior endplate L5. Most likely this also resulted in some stimulation of osteophytic growth from the posterior ring apophysis as both bone and scar tissue was encountered behind the interbody fusion cage during the endoscopic decompression following the L5/S1 VARILIF-L™. The bone graft chamber was filled with bone without any evidence of instability suggesting that standalone expandable implants without supplemental posterior pedicle screw fixation may fuse successfully but may still result in recurrent radicular symptoms due to the kind of problems encountered in this patient. Whether these occurred because of the endoscopic approach or the transforaminal cage implantation is unclear. Likely, it is more related to the fusion cage rather than the combination of the two factors since symptomatic scar tissue formation in the hidden zone of Macnab after routine endoscopic decompression is uncommon.
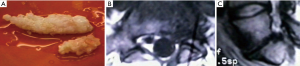
This case report calls attention to the fact that standalone expandable interbody fusion cages may produce unintended consequences that may require additional interventional or surgical aftercare, including revision surgery. As demonstrated by this case example, careful preoperative workup may identify the new pain generator that may develop months after the index surgery after an intermediate symptom-free interval. Bony and scar tissue formation behind the interbody fusion cage as well as bulging of remaining annular tissue may procedure painful nerve root compression or tethering. Open surgery may be required, but as this case demonstrates, endoscopic revision decompression can be useful.
Conclusions
Standalone outpatient lumbar transforaminal endoscopic interbody fusion with an expandable threaded cylindrical cage may produce subsidence-induced recurrence of sciatica-type back and leg symptoms long after the index endoscopic fusion surgery. A symptom-free interval may ensure before radicular symptoms may recur. If the patient’s symptoms cannot be managed non-operatively, repeat endoscopic decompression is feasible and should be considered along with other types of open or minimally invasive translaminar surgeries after careful analysis all available clinical information.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Alvi MA, Kurian SJ, Wahood W, et al. Assessing the Difference in Clinical and Radiologic Outcomes Between Expandable Cage and Nonexpandable Cage Among Patients Undergoing Minimally Invasive Transforaminal Interbody Fusion: A Systematic Review and Meta-Analysis. World Neurosurg 2019;127:596-606.e1. [Crossref] [PubMed]
- Schmoelz W, Sandriesser S, Loebl O, et al. Effect of cage design, supplemental posterior instrumentation and approach on primary stability of a lumbar interbody fusion - A biomechanical in vitro study. Clin Biomech (Bristol, Avon) 2017;48:30-4. [Crossref] [PubMed]
- Lewandrowski KU, Ransom NA, Ramírez León JF, et al. The Concept for A Standalone Lordotic Endoscopic Wedge Lumbar Interbody Fusion: The LEW-LIF. Neurospine 2019;16:82-95. [Crossref] [PubMed]
- Regev GJ, Lador R, Salame K, et al. Minimally invasive spinal decompression surgery in diabetic patients: perioperative risks, complications and clinical outcomes compared with non-diabetic patients' cohort. Eur Spine J 2019;28:55-60. [Crossref] [PubMed]
- Lewandrowski KU. Surgical Technique of Endoscopic Transforaminal Decompression and Fusion with a Threaded Expandable Interbody Fusion Cage and A Report of 24 Cases. J Spine 2018;7:409. [Crossref]
- Lewandrowski KU. “Outside-in” technique, clinical results, and indications with transforaminal lumbar endoscopic surgery: a retrospective study on 220 patients on applied radiographic classification of foraminal spinal stenosis. Int J Spine Surg 2014. [Crossref] [PubMed]
- Yeung AT. Lessons Learned from 27 Years’ Experience and Focus Operating on Symptomatic Conditions of the Spine under Local Anesthesia: The Role and Future of Endoscopic Spine Surgery as a “Disruptive Technique” for Evidenced Based Medicine. J Spine 2018;7:413. [Crossref]
- Hoogland T, Schubert M, Miklitz B, et al. Transforaminal posterolateral endoscopic discectomy with or without the combination of a low-dose chymopapain: a prospective randomized study in 280 consecutive cases. Spine (Phila Pa 1976) 2006;31:E890-7. [Crossref] [PubMed]
- Ruetten S, Komp M, Merk H, et al. Recurrent lumbar disc herniation after conventional discectomy: a prospective, randomized study comparing full-endoscopic interlaminar and transforaminal versus microsurgical revision. J Spinal Disord Tech 2009;22:122-9. [Crossref] [PubMed]
- Sengupta DK, Herkowitz HN. Lumbar spinal stenosis. Treatment strategies and indications for surgery. Orthop Clin North Am 2003;34:281-95. [Crossref] [PubMed]
- Yeung AT, Roberts A, Zhu L, et al. Treatment of Soft Tissue and Bony Spinal Stenosis by a Visualized Endoscopic Transforaminal Technique Under Local Anesthesia. Neurospine 2019;16:52-62. [Crossref] [PubMed]
- Huskisson EC, Jones J, Scott PJ. Application of visual-analogue scales to the measurement of functional capacity. Rheumatol Rehabil 1976;15:185-7. [Crossref] [PubMed]
- Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976) 2000;25:2940-52; discussion 2952. [Crossref] [PubMed]
- Lewandrowski KU. Successful outcome after outpatient transforaminal decompression for lumbar foraminal and lateral recess stenosis: The positive predictive value of diagnostic epidural steroid injection. Clinical Neurology and Neurosurgery 2018;173:38-45. [Crossref] [PubMed]
- Lewandrowski KU. Retrospective analysis of accuracy and positive predictive value of preoperative lumbar MRI grading after successful outcome following outpatient endoscopic decompression for lumbar foraminal and lateral recess stenosis. Clin Neurol Neurosurg 2019;179:74-80. [Crossref] [PubMed]
- Yeung AT, Lewandrowski KU. Retrospective analysis of accuracy and positive predictive value of preoperative lumbar MRI grading after successful outcome following outpatient endoscopic decompression for lumbar foraminal and lateral recess stenosis. Clin Neurol Neurosurg 2019;181:52. [Crossref] [PubMed]
- Yeung A, Lewandrowski KU. Five-year clinical outcomes with endoscopic transforaminal foraminoplasty for symptomatic degenerative conditions of the lumbar spine: a comparative study of inside-out versus outside-in techniques. J Spine Surg 2020;6:S66-S83.
- Lewandrowski KU. Incidence, Management, and Cost of Complications After Transforaminal Endoscopic Decompression Surgery for Lumbar Foraminal and Lateral Recess Stenosis: A Value Proposition for Outpatient Ambulatory Surgery. Int J Spine Surg 2019;13:53-67. [Crossref] [PubMed]
- Lewandrowski KU. Readmissions After Outpatient Transforaminal Decompression For Lumbar Foraminal And Lateral Recess Stenosis. Int J Spine Surg 2018;12:342-51. [Crossref] [PubMed]


