
Computer-assisted navigation in complex cervical spine surgery: tips and tricks
Introduction
The intimate relationship between the cervical vertebra and its surrounding neurovascular structures creates inherent surgical challenges when operating on the cervical spine. Accurate placement of spinal implants is critically important to avoid iatrogenic complications and costly returns to the operative suite. Screw breach places nearby structures at risk, which has spurred critical appraisal of screw placement using volumetric imaging (1). In an effort to improve accuracy, there has been a surge of image-guided technology. As a result, the use of stereotactic navigation has become increasingly more mainstream. This is especially the case when usual anatomic landmarks cannot reliably orient the surgeon intraoperatively, as is the case with significant trauma, severe degeneration, or developmental malformations.
The first iterations of the technology relied on preoperatively acquired computed tomography (CT) scans to map the patient anatomy (2-4). Intraoperatively, the CT images were loaded to a navigation station and the digital three-dimensional (3D) model was referenced to the patient’s anatomy using a guided probe touching specific anatomic landmarks (e.g., spinous processes) or externally applied reference markers. This referencing method was termed the “point merge technique”. The protocol worked well for cranial surgery when the software needed only register an immobile skull fixed in a rigid external frame. However, as one would expect, alterations in spine position on the operating table created considerable registration errors and instrument inaccuracy when this technology was applied to spine surgery (5). To compensate for changes in vertebrae position, each level of interest would need to be registered individually, adding substantial time to the navigation set up. The advent of intraoperatively acquired imaging dramatically improved accuracy and registration times, transforming the technology from novelty to standard practice. Now navigation systems use frameless, integrated registration processes, which have reduced the time to place an image-guided pedicle screw in half (5).
The literature describes several freehand and fluoroscopically-guided methods of screw placement (6-8), with reported rates of pedicle wall violation ranging from 5.2–54.7% (9-11). Though errantly placed screws are rarely clinically relevant (<5%), in the cervical spine the screws place vital neurovascular structures at risk (9,12-16). Computer-navigation is shown to decrease rates of pedicle wall violation, lower operative times, and decrease the rate of revision procedures (11,17-24). Despite the improved accuracy, it is important to note that no form of navigation has proven to decrease neurologic or vascular complications, increase fusion rates, or improve pain or health outcome scores (10,18,25).
This article describes the practicalities of stereotactic navigation, details our methods for registration and direct referencing, and shares tips on best practices for this burgeoning technology, all with a focus on complex cervical spine surgery using the Medtronic O-arm and StealthStation (Medtronic, Minneapolis, MN, USA). Several other systems are available including Iso-C C-arm (Siremobil Iso-C 3D; Siemens Medical Solutions, Erlangen, Germany) and NaviVision (VectorVision, BrainLab, Germany), and our discussion should be generally applicable as, to date, the systems have shown no differences in pedicle screw placement accuracy (22,26-28). Additionally, many instrumentation systems have been FDA cleared for use with Medtronic’s Navlock Tracker system on its StealthStation including Alphatec Spine Inc., Globus Medical Inc., Orthofix Inc., among others. Each system has its pros and cons, and every surgeon has his or her individual preferences, but the principles remain constant. The O-arm and SteathStation are only highlighted due to our familiarity with the systems rather than any superiority over other commercially available systems.
How it works
Stereotactic navigation systems will use CT or pulsed fluoroscopic images (obtained either preoperatively or intraoperatively) and an image processing software to generate a volumetric model of the patient’s anatomy. Both two-dimensional (2D) and 3D projections are available as the surgeon and case requires. For 3D modelling, a series of pulsed X-ray exposures are collected by an image intensifier that spins 360 degrees around the patient, and from these images a reconstruction algorithm generates a 3D model. The resolution of the voxel rendered image depends on several factors including the image frequency and intensity and rotational speed of the X-ray source.
Prior to image acquisition, a reference frame is rigidly positioned with respect to the patient’s anatomy to permit the navigation station to correlate the 3D image to points with the patient’s position in space. Optical or electromagnetic (EM) localization is used to detect the frame. With optical tracking, a camera detects infrared light from optical markers (either reflective spheres or light-emitting diodes attached to the instruments and reference frame). Once infrared light is emitted by the camera and reflected off the spheres (or emitted directly by LEDs), the system uses two camera lenses to geometrically triangulate the spatial coordinates of each optical marker and transmits the data to the navigation software for computation. EM tracking works similarly but uses an emitter, which emits a low-energy magnetic field with unique field properties at every coordinate within the field. The instruments contain EM sensors which allow the navigation software to identify the instrument’s location within the field. Spine cases will normally employ optical tracking rather than EM, as the navigation field for optical tracking is much larger than that for EM tracking.
After receiving the localization data, the navigation station processes the sensor data in real-time to compute the position and angle of the surgical instruments in relation to the registered model. For the software to correctly display the instrument’s spatial location, the software must create a map between points on the patient and points in the images. This process is called registration. After registration is complete, the computer uses the created map to identify corresponding points between the image and patient. The navigation station can then display the data in several forms, including simultaneous axial, coronal, and sagittal images; 3D models; or 2D projections analogous to fluoroscopy.
Method for employing stereotactic navigation
Room set up
The operating table must be selected to be compatible with the image acquisition system. In this case, a radiolucent table with table supports at the head and foot are used which allows the O-arm gantry to pass around a patient and close the telescoping door (i.e., Jackson or Allen table). For prone positioning, the patient’s arms are secured to their sides to provide additional room at the head of the bed. Whereas securing a patient’s arms at their sides typically diminishes resolution of 2D radiography, 3D acquired images are protected from this image degradation. If there is concern about having sufficient space at the head of the bed, the O-arm should be tested before prepping to confirm that the appropriate placement is possible.
Next, attention should be turned toward room layout. The O-arm, navigation station, and StealthStation take up considerably more room than a standard fluoroscopy unit, and therefore, image-guided cases are ideally performed in larger operative suites. For cervical cases, the StealthStation is preferably placed at the head of the bed to ensure the reference frame and instruments fall within the ideal navigation field (between 0.95 and 2.4 meters). However, if the angle of approach for instrumentation favors the sensors at the foot of the bed, this would supersede convention. Ideally, the passive reference frame is transfixed on the side of the wound nearest the sensor. This prevents obstructing the line-of-sight between the camera and reference frame when using instruments within the navigation field. The O-arm should remain on the side closest to the door, so it may be removed when not in use. It need only be present for a brief time during image acquisition, and therefore, a single O-arm can support several simultaneous stereotactic navigation cases if separate navigation stations are available.
Tracked instruments and the reference frame
With any image-guided system, special instruments are needed, and costs scale with the number used due to the disposable tracking spheres that must be attached. We generally use the following tracked instruments for pedicle screw placement: drill, tap, ball-tip probe, and screw driver. For optical tracking, each instrument has a unique array where reflective spheres are secured, which the infrared camera then uses to track. These balls must be firmly set in place (confirmed by a click). If not fully seated, the tracking software will be unable to register or track the array. If the spheres become dirtied, the infrared light will no longer reflect and the tracking will fail. If blood covers a sphere, wipe it with a moist sponge followed by a dry one to restore its reflective surface.
Selecting the method of transfixing the passive reference array is critically important to ensure accurate registration of patient anatomy without obstructing the surgical field. For cases involving the upper cervical spine, we prefer to use a Mayfield attachment (Figure 1) when possible as this provides a rigid position in relation to the spine while remaining out of the surgical field. The non-sterile post is covered with a sterile clear plastic drape then the remainder of the draping is performed as usual. The outer drapes are cut over the sterilely-draped post with scissors that are passed off the field. The blue drape is secured to the post with rubber bands and the frame is inserted through the clear plastic drape into the post. If direct fixation to the skeleton is required, the frame can be placed on the spinous process of C2 for higher accuracy at the cost of crowding the field. Alternatively, a spinous process clamp on T1 or T2 can be used for lower cervical levels. The spinous process clamp minimizes the distance of the reference frame to instrumented levels which optimizes accuracy, yet the proximity of the frame to the working field creates new challenges. Instruments will have to circumvent the frame while working in the wound, and if the frame is bumped, the accuracy may degrade.

Before securing the reference frame to the patient, register the instruments with the unsecured passive frame. Registering the instruments after image acquisition introduces risk of inaccuracy if the reference frame or patient anatomy is inadvertently displaced by the process. Because instrument registration confirms a known spatial relationship between the frame and instrument arrays, the frame does not need to be fixed to the patient. Thus, the surgical scrub can register instruments at any time after the StealthStation is set up.
Wound management
The entire exposure should be performed before securing the passive frame. The surgeon should confirm the infrared lenses can visualize the frame and instruments at their desired levels and trajectories. If the frame and instruments obstruct each other or the lenses cannot identify them individually, the frame must be adjusted or moved to a different spinous process.
The deep retractors can remain in the wound throughout the case, including image acquisition. Leaving the retractors in the wound limits the risk of inadvertently bumping the reference frame while replacing them, saves some time, and avoids potential motion within the registered anatomy. However, this constant retraction risks potential tissue necrosis and the retained retractors will blemish images with metal artifact. When placing or removing retractors, avoid contacting the passive frame to preserve the fidelity of the system’s map. Special care should be taken when the passive frame post is located at the apex of the skin incision. The retracted tissues can tension the apex which will shorten the length of the wound and distort the position of the spinous process clamp after image acquisition.
Direct referencing
For cases involving multiple levels or spinal instability, we use a “direct referencing” technique in order to repeatedly verify accuracy of the map anatomy. To do so, we place 1.8 mm cranial plate fixation screws strategically on lamina across the planned surgical levels. These act as fiducial markers. The locations are recorded and the screws removed before decortication or wound closure. While navigating, the screws create easily identifiable and reliable reference points. The fiducial markers should be used to verify proper orientation of the images and accuracy of the instruments (Figure 2). The system uses dynamic referencing and will constantly recompute instrument location using the reference frame. Throughout the case, and particularly before key portions of instrumentation, verify the accuracy and responsiveness of the tracking system by using the probe to touch bony landmarks or fiducial markers at various points in the field. Confirm these points correspond to the correct position on the imaged model. Should the accuracy degrade, the surgeon should pause and re-register the system, abandon the process, or use additional confirmatory imaging with fluoroscopy. Occasionally, moving the reference frame closer to the vertebrae of interest may improve accuracy.
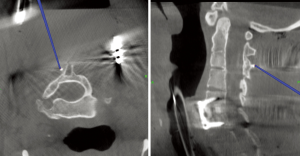
Image acquisition
There are several techniques to prepare the surgical field to ensure sterility and improve registration accuracy. Commercial drapes are available for the O-arm to maintain sterility during gantry positioning, but they incur additional cost. We employ an alternative method to protect the sterile field. First, the wound is filled with sterile saline to prevent tissue desiccation and minimize air-tissue contrast within the image. Two three-quarter drapes are placed over the patient, slightly overlapping and connected together at midline with staples or clips. The reference array is excluded from this draping and sticks out from between the two drapes. A third drape or towel covers the reference frame while the gantry positions itself and telescoping door closes around the patient. This should be removed, and gloves changed, to reveal the reference frame prior to image acquisition. After all images are acquired and the O-arm removed, the protective drapes are separated and discarded, taking caution not to contaminate the field. Again, gloves should be changed after touching these protective drapes. This setup preserves sterile environment below the drape.
With the O-arm in position, 2D scout images are obtained to confirm the gantry is properly positioned. If the levels of interest cannot be captured in a single spin, then multiple spins can be performed without changing the draping or frame. The occiput to T2 levels typically requires no more than two spines. Spins should marginally overlap to guarantee that all pedicles and lateral masses are captured. The O-arm has two settings for image quality: high-definition (HD) and standard. We use HD modes with larger patients, when metallic implants are already present, when retractors remain in place during image acquisition, or when working at the occipitocervical or cervicothoracic junctions. In standard mode, the rotor spins the X-ray source at 30 degrees per second, acquiring images at 30 frames per second. In HD mode, the rotor spins at 15 degrees per second, effectively doubling the exposure dose. If the recommended HD 3D dosing is selected for a large patient (120 kVp, 240 mAs) for the smallest field of view (20 cm), this will result in an exposure of approximately 38 mGy. However, most other protocols range from 10 to 20 mGy depending on the field and dosing. If radiation exposure is a concern, a low dose mode is available that will decrease the dosing by 35% from the standard protocol. Once the image quality is selected, anesthesia should hold respirations during image acquisition (usually 14–28 seconds). After image acquisition, the O-arm can be removed from the suite.
Image-guided instrumentation
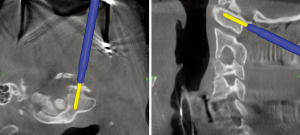
After constructing the navigated image, instrumentation can proceed. Decompression is deferred until after instrumentation (or at least after preparing the screw holes) because decompression initiates new bony bleeding, exposes vulnerable neural structures, and risks decreased navigation accuracy from displacing the spine from its imaged position. Using anatomic landmarks, select a desired start point and use the navigated ball-tip probe to confirm the proper trajectory and depth to pass the drill through the pedicle and into the vertebral body (seen on sagittal and axial planes) (Figure 3). A projection from the tip of the instrument assists visualization of the path the instrument will take during advancement. Images are displayed on overhead monitors or directly on the navigation station within clear view of the surgeon while using the instruments.

The StealthStation screen can display up to four separate images simultaneously (axial, sagittal, probe’s eye, 2D fluoroscopy, or 3D model). We routinely use the axial and sagittal views (Figure 4). The axial view will confirm the appropriate start point, guide midline angulation, and help estimate depth. The sagittal view will assist in centering the tool within the pedicle. The probe’s eye view is the least helpful, but it shows a composite view in the coronal plane along the axis of the instrument. Finally, the 2D and 3D model views provide a more global assessment.
After an appropriate start point is identified with the ball-tip probe, the dorsal cortex is burred to create a pilot hole. The drill tip is positioned in the pilot hole. As the drill advances, it is tracked on the navigated image in real-time so minor adjustments can be made to center the drill within the pedicle. The mapping software can modify the image to overlay a simulated pedicle screw, which confirms the length and diameter of the screw. On the navigation system, the length of the prepared screw track is measured by placing one cursor at the start point and another at the preferred depth of insertion producing a display of the linear distance between them. If the images differ from the preoperative plan or intraoperative landmarks, the preoperative measurements are preferentially used or position and depth are verified with fluoroscopic imaging.
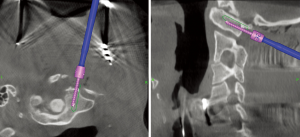
We use a standard ball-tip probe to confirm the five walls were not breeched by the drill (lateral, medial, inferior, superior, and the screw hole floor). The navigated ball-tip probe can confirm drill hole depth is appropriate. The hole is then tapped using a navigated tap, one millimeter undersized from the intended screw. The probe again confirms intact walls. The screw is then placed using a navigated driver (Figure 5). Both handheld and powered drivers are available. Powered insertion provides a steadier navigated image, however current drivers are off-the-shelf systems that have been adapted to attach a navigation array. As such, these modified drivers tend to be awkward and unwieldy.
While inserting the screw, attention is given to the capture between the driver and the screw head because it can loosen during screw insertion and produce an inaccurate display of the screw position. The connection between the screw and the shaft of the driver is routinely retightened during insertion. After completion of instrumentation, standard 2D fluoroscopic images confirm proper screw position so any hardware complications can be identified and addressed before closure. Typically, an O-arm spin after instrumentation is not required unless a screw is close to a critical structure or the native anatomy is so distorted that fluoroscopic imaging is ineffective.
Other indications
Beyond screw insertion, computer-assisted navigation can be utilized in a number of other ways to aide complicated spine procedures. The ball-tip probe is commonly used to verify adequate decompression and to localize and measure anatomic structures. Stereotactic navigation has found a role in both short and long segment instrumentation. In some studies, it has been shown to shorten implantation times and decrease blood loss (17,29). Additionally, stereotactic navigation clearly improves implantation accuracy. This is most notable within the thoracic spine, where pedicle breeches are reported as high as 47% (11,13,18,22,30). These benefits are offset by increased radiation exposure to the patient and higher capital costs compared to standard fluoroscopic or freehand techniques. Though the surgical team benefits from lessened radiation exposure, the patient on average is subject to an effective radiation dose of 6 mSv. Fortunately, this is a low dose exposure and should not pose a specific carcinogenic risk (31,32). However, a standard abdominal CT is ~8 mSv, and, by epidemiologic data, is correlated with a small cancer risk. Therefore, the surgeon should include an honest discussion of radiation risks in the informed consent if the O-arm will be utilized.
Summary and case example
A 25-year-old patient with Klippel-Feil Syndrome presented to our clinic with signs and symptoms of severe progressive cervical myelopathy. Imaging showed numerous formation and segmentation abnormalities, as well as anomalous vertebral artery anatomy. We undertook a circumferential cervical decompression and fusion consisting of C5–6 anterior cervical discectomy and fusion surgery (ACDF) and posterior C1–T2 decompression and fusion. We summarize below our steps with regards to use of stereotactic navigation in this complex case:
- reference frame secured opposite to the Mayfield clamp (Figure 1);
- after exposure, O-arm brought in for image acquisition (Figure 3);
- identification of fiducial markers to verify accuracy (Figure 2);
- navigated drill used to confirm screw start point, followed by projected drill track and screw placement (Figure 5);
- intraoperative fluoroscopic imaging showed screw placement along projected track.
Conclusions
Stereotactic navigation is a burgeoning technology that has a proven benefit in certain situations. Provided is a historical, theoretical, and methodological background to permit an informed decision about using image-guidance in practice. Navigated surgery requires constant vigilance. Surgeons should not fall into a state of complacency when using navigated instruments. Repeated accuracy checks with direct referencing screws should be employed to verify the navigation mapping has not degraded. If images deviate from the preoperative plan or intraoperative landmarks, the system must be re-registered or abandoned. Employed correctly, stereotactic navigation is a powerful tool in complex cervical cases, as described here, where traditional techniques fall short.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lee A. Tan and Ilyas S. Aleem) for the series “Advanced Techniques in Complex Cervical Spine Surgery” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: The series “Advanced Techniques in Complex Cervical Spine Surgery” was commissioned by the editorial office without any funding or sponsorship. ISA served as the unpaid Guest Editors of the series “Advanced Techniques in Complex Cervical Spine Surgery” published in Journal of Spine Surgery. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gertzbein SD, Robbins SE. Accuracy of pedicular screw placement in vivo. Spine (Phila Pa 1976) 1990;15:11-4. [Crossref] [PubMed]
- Austin MS, Vaccaro AR, Brislin B, et al. Image-guided spine surgery: a cadaver study comparing conventional open laminoforaminotomy and two image-guided techniques for pedicle screw placement in posterolateral fusion and nonfusion models. Spine (Phila Pa 1976) 2002;27:2503-8. [Crossref] [PubMed]
- Lavallée S, Sautot P, Troccaz J, et al. Computer-assisted spine surgery: a technique for accurate transpedicular screw fixation using CT data and a 3-D optical localizer. J Image Guid Surg 1995;1:65-73. [Crossref] [PubMed]
- Merloz P, Tonetti J, Pittet L, et al. Computer-assisted spine surgery. Comput Aided Surg 1998;3:297-305. [Crossref] [PubMed]
- Zhang W, Takigawa T, Wu Y, et al. Accuracy of pedicle screw insertion in posterior scoliosis surgery: a comparison between intraoperative navigation and preoperative navigation techniques. Eur Spine J 2017;26:1756-64. [Crossref] [PubMed]
- Krag MH, Weaver DL, Beynnon BD, et al. Morphometry of the thoracic and lumbar spine related to transpedicular screw placement for surgical spinal fixation. Spine (Phila Pa 1976) 1988;13:27-32. [Crossref] [PubMed]
- Krag MH. Biomechanics of thoracolumbar spinal fixation. A review. Spine (Phila Pa 1976) 1991;16:S84-99. [Crossref] [PubMed]
- Vaccaro AR, Rizzolo SJ, Allardyce TJ, et al. Placement of pedicle screws in the thoracic spine. Part I: morphometric analysis of the thoracic vertebrae. J Bone Joint Surg Am 1995;77:1193-9. [Crossref] [PubMed]
- Kim YJ, Lenke LG, Bridwell KH, et al. Free hand pedicle screw placement in the thoracic spine: is it safe? Spine (Phila Pa 1976) 2004;29:333-42; discussion 342. [Crossref] [PubMed]
- Amiot LP, Lang K, Putzier M, et al. Comparative results between conventional and computer-assisted pedicle screw installation in the thoracic, lumbar, and sacral spine. Spine (Phila Pa 1976) 2000;25:606-14. [Crossref] [PubMed]
- Shin BJ, James AR, Njoku IU, et al. Pedicle screw navigation: a systematic review and meta-analysis of perforation risk for computer-navigated versus freehand insertion. J Neurosurg Spine 2012;17:113-22. [Crossref] [PubMed]
- Hardin CA, Nimjee SM, Karikari IO, et al. Percutaneous pedicle screw placement in the thoracic spine: a cadaveric study. Asian J Neurosurg 2013;8:153-6. [Crossref] [PubMed]
- Hart RA, Hansen BL, Shea M, et al. Pedicle screw placement in the thoracic spine: a comparison of image-guided and manual techniques in cadavers. Spine (Phila Pa 1976) 2005;30:E326-31. [Crossref] [PubMed]
- Schatlo B, Molliqaj G, Cuvinciuc V, et al. Safety and accuracy of robot-assisted versus fluoroscopy-guided pedicle screw insertion for degenerative diseases of the lumbar spine: a matched cohort comparison. J Neurosurg Spine 2014;20:636-43. [Crossref] [PubMed]
- Fraser J, Gebhard H, Irie D, et al. Iso-C/3-dimensional neuronavigation versus conventional fluoroscopy for minimally invasive pedicle screw placement in lumbar fusion. Minim Invasive Neurosurg 2010;53:184-90. [Crossref] [PubMed]
- Gruenberg M, Petracchi M, Valacco M, et al. The influence of anatomy (normal versus scoliosis) on the free-hand placement of pedicle screws: Is misplacement more frequent in patients with anatomical deformity? Evid Based Spine Care J 2010;1:11-7. [Crossref] [PubMed]
- Sasso RC, Garrido BJ. Computer-assisted spinal navigation versus serial radiography and operative time for posterior spinal fusion at L5-S1. J Spinal Disord Tech 2007;20:118-22. [Crossref] [PubMed]
- Gelalis ID, Paschos NK, Pakos EE, et al. Accuracy of pedicle screw placement: a systematic review of prospective in vivo studies comparing free hand, fluoroscopy guidance and navigation techniques. Eur Spine J 2012;21:247-55. [Crossref] [PubMed]
- Mirza SK, Wiggins GC, Kuntz C 4th, et al. Accuracy of thoracic vertebral body screw placement using standard fluoroscopy, fluoroscopic image guidance, and computed tomographic image guidance: a cadaver study. Spine (Phila Pa 1976) 2003;28:402-13. [Crossref] [PubMed]
- Rajasekaran S, Vidyadhara S, Ramesh P, et al. Randomized clinical study to compare the accuracy of navigated and non-navigated thoracic pedicle screws in deformity correction surgeries. Spine (Phila Pa 1976) 2007;32:E56-64. [Crossref] [PubMed]
- Laine T, Lund T, Ylikoski M, et al. Accuracy of pedicle screw insertion with and without computer assistance: a randomised controlled clinical study in 100 consecutive patients. Eur Spine J 2000;9:235-40. [Crossref] [PubMed]
- Noriega DC, Hernández-Ramajo R, Rodríguez-Monsalve Milano F, et al. Risk-benefit analysis of navigation techniques for vertebral transpedicular instrumentation: a prospective study. Spine J 2017;17:70-5. [Crossref] [PubMed]
- Watkins RG, Gupta A, Watkins RG. Cost-effectiveness of image-guided spine surgery. Open Orthop J 2010;4:228-33. [Crossref] [PubMed]
- Dea N, Fisher CG, Batke J, et al. Economic evaluation comparing intraoperative cone beam CT-based navigation and conventional fluoroscopy for the placement of spinal pedicle screws: a patient-level data cost-effectiveness analysis. Spine J 2016;16:23-31. [Crossref] [PubMed]
- Verma R, Krishan S, Haendlmayer K, et al. Functional outcome of computer-assisted spinal pedicle screw placement: a systematic review and meta-analysis of 23 studies including 5,992 pedicle screws. Eur Spine J 2010;19:370-5. [Crossref] [PubMed]
- Silbermann J, Riese F, Allam Y, et al. Computer tomography assessment of pedicle screw placement in lumbar and sacral spine: comparison between free-hand and O-arm based navigation techniques. Eur Spine J 2011;20:875-81. [Crossref] [PubMed]
- Nooh A, Lubov J, Aoude A, et al. Differences between manufacturers of computed tomography-based computer-assisted surgery systems do exist: a systematic literature review. Global Spine J 2017;7:83-94. [Crossref] [PubMed]
- Lee GY, Massicotte EM, Rampersaud YR. Clinical accuracy of cervicothoracic pedicle screw placement: a comparison of the "open" lamino-foraminotomy and computer-assisted techniques. J Spinal Disord Tech 2007;20:25-32. [Crossref] [PubMed]
- Assaker R, Reyns N, Vinchon M, et al. Transpedicular screw placement: image-guided versus lateral-view fluoroscopy: in vitro simulation. Spine (Phila Pa 1976) 2001;26:2160-4. [Crossref] [PubMed]
- Waschke A, Walter J, Duenisch P, et al. CT-navigation versus fluoroscopy-guided placement of pedicle screws at the thoracolumbar spine: single center experience of 4,500 screws. Eur Spine J 2013;22:654-60. [Crossref] [PubMed]
- Nelson EM, Monazzam SM, Kim KD, et al. Intraoperative fluoroscopy, portable X-ray, and CT: patient and operating room personnel radiation exposure in spinal surgery. Spine J 2014;14:2985-91. [Crossref] [PubMed]
- Vrijheid M, Cardis E, Blettner M, et al. The 15-Country Collaborative Study of Cancer Risk Among Radiation Workers in the Nuclear Industry: design, epidemiological methods and descriptive results. Radiat Res 2007;167:361-79. [Crossref] [PubMed]


