
Complications following posterior cervical decompression and fusion: a review of incidence, risk factors, and prevention strategies
Introduction
Symptomatic cervical spine pathologies are becoming increasingly prevalent with the aging world population. Posterior cervical decompression and fusion (PCF) is a common surgical technique used to treat various cervical spine pathologies (Figure 1). However, post-operative complications associated with PCF can negatively impact patient outcome.
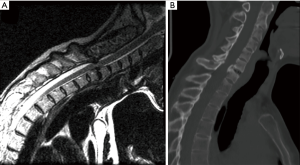
In this review article, we aim to accomplish the following goals: (I) evaluate the current evidence regarding the incidence of short- and long-term complications in patients undergoing posterior cervical fusion, including clinical complications such as C5 palsy and surgical site infection (SSI), as well as radiographic complications such as adjacent segment degeneration and junctional kyphosis; (II) identify and review the risk factors for postoperative complications associated with PCF; and (III) examine and review the various prevention strategies for common PCF complications.
Methods
We performed a comprehensive review of the currently available English literature published regarding posterior cervical spine surgery using three online databases: PubMed, Cochrane Database of Systematic Reviews, and Google Scholar. Search terms used included combinations of “posterior cervical fusion”, “complication”, “adjacent segment degeneration”, “neurologic deficit”, “C5 palsy”, “junctional kyphosis”, “durotomy”, “dural tear”, and “pseudoarthrosis”. All titles obtained using this search query were screened to identify relevant articles. Articles describing posterior cervical constructs whose principal focus of addressing thoracolumbar deformity were excluded. The remainder were reviewed in their entirety and the references of these articles were searched to identify further relevant studies. In the case of studies comparing different modalities of treatment, such as anterior cervical discectomy and fusion (ACDF) versus PCF, data from the relevant subgroups were identified for inclusion in the review, and the remainder was discarded. Data relevant to the research question was recorded in tabular form.
Results
Overall complication rate
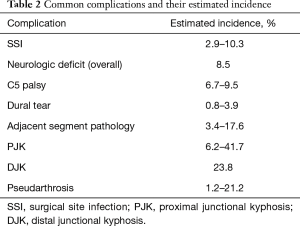
The postoperative complication rates reported in the literature have varied significantly ranging from 8.6% to 49.1%. However, most studies have demonstrated that the incidence is likely between 15% and 25% (1-13) (Table 1). A recently published meta-analysis of 31 studies with a minimum follow-up of 12 months, which primarily consisted of small, single-center retrospective studies, reported a 9.0% complication rate (5). Of note, the only prospective study to directly examine perioperative complication following PCF found that 26 of the 53 patients (49.1%) in their cohort developed either a minor or major complication, significantly higher than previously reported values (3). The most common complications reported across the literature include acute blood loss anemia requiring postoperative transfusion, SSI, C5 palsy or other transient neurologic deficit, incidental durotomy, and pseudoarthrosis (Table 2).

Full table
Full table
In comparisons with ACDF, PCF is associated with greater perioperative morbidity and mortality overall. Leckie et al. analyzed a cohort of 1,269 patients undergoing either primary or revision cervical spine fusion, using anterior, posterior, or combined approach. PCF was associated with a 22.5% complication rate, compared to 7.5% in anterior fusions. Delirium and draining wounds comprised the most common adverse postoperative events, occurring in 16.7% and 11.7% of patients, respectively (11). Likewise, Badhiwala et al. conducted a propensity score-matched analysis of data from the National Inpatient Sample (NIS), finding that PCF was associated with a higher rate of various complications including myocardial infarction (MI), pulmonary embolism (PE), and deep vein thrombosis (DVT) (6). Other studies using NIS data found that PCF was associated with over three times more complications than anterior cervical procedures, 15.4% vs. 4.1%, and a higher rate of mortality, 1.4% vs. 0.3% (4,13). The authors note, however, that patients included in the NIS who underwent PCF were significantly older and had a greater comorbidity index than their counterparts undergoing ACDF, thus there was patient selection bias which increased their inherent risk of postoperative complication.
A variety of risk factors have been identified to contribute to the overall complication. Among the most widely reported include older age and frailty (1,2,4,9-11). A study by Shin et al. found increasing frailty, as measured by the modified frailty index (mFI) to be significantly associated with any complication, mortality, and Clavien-Dindo grade IV complications, which are life-threatening conditions involving single or multiorgan dysfunction that require intermediate or intensive care. Higher frailty scores were associated with 41.3× greater odds of Clavien class IV complications in particular (9). Likewise, impaired functional status has been linked to poor postoperative outcomes. In a study by DePasse et al., partial or complete dependence on others was associated with a complication rate of over 26%, and in another by Badiee et al., loss of independent ambulation was associated with more than double the odds of 30-day medical complication (1,10).
Increased number of fusion levels was also associated with increased risk of postoperative complication, though primarily in unadjusted analyses (2,7,10,11). In one study, fusion of more than one level was associated with 40% greater odds of complication and a significant increase in the incidence of postoperative blood transfusion. However, this study did not include a multivariate analysis to adjust for other contributing factors (10). Similarly, Medvedev et al. found a nonlinear association on univariate comparison, with a complication rate of 35.6% on single level fusion compared to 46.2% for six or more levels, but this difference attenuated after adjusting for other factors. Other studies have suggested a mechanism for this association, namely that increasing the number of fused levels lead to increased blood loss and operative times, which in turn adversely impacts patients’ postoperative risk of morbidity (1,7). Intraoperative blood loss greater than 500 mL has been associated with up to 3.67× greater odds of postoperative complication (1).
Diabetes is a commonly cited risk factor for adverse postoperative outcomes as well (2,10,14). In patients with cervical spondylotic myelopathy (CSM), regardless of surgical approach, diabetes was found to be an independent predictor of unplanned intubation, ventilator use >48 hours, DVT or thrombophlebitis, and urinary tract infection, with greater risk conferred by insulin dependence compared to medication- or diet-controlled diabetes (15). Similarly, smoking has been found to cause poor postoperative outcomes in other spine surgeries, an association that holds with PCF as well (2,10). Other risk factors that have been inconsistently reported to affect overall complication risk after PCF include body mass index (BMI) >35 (2), preoperative narcotic use (1), male sex (2,4), and the American Society of Anesthesiologists (ASA) score above 3 (2).
SSI incidence and prevention
SSI is among the most common postoperative complications associated with PCF. Systemic antibiotic therapy and surgical wound debridement are often necessary. Infections within the acute to subacute postoperative period are typically caused by skin flora, such as Staphylococcus aureus, Staphylococcus epidermidis, and group A Streptococcus. Late presenting infections can be caused by other skin flora with lower virulence, such as Propionibacterium. Gram-negative bacilli, including Klebsiella, Escherichia coli, Pseudomonas, and Proteus, are uncommon pathogens but can be found in intravenous (IV) drug users (16).
The 30-day incidence of these infections is typically reported to be between 2% to 10%, with most retrospective studies reporting values between 2% to 4% (1,10,13,17-19). One study by Strom et al. found that in a cohort of 92 patients, the 1-year incidence of SSI was 10.9% before infection prevention interventions were enacted (20). Superficial infections, those that affect the skin and subcutaneous layers, have been reported to occur in about 1.5–2.0% of patients. Deep infections, which disseminate deep to the fascia and can affect deeper structures, are found at about the same rate (1,11). These infections can significantly increase the cost associated with PCF surgery, increasing hospital stays by an average of 3–7 days (10) and contributing a mean increase of $4,067 in the cost of care (21). However, given that patients often present with nonspecific symptoms such as fever and pain at the incision site, diagnosis is delayed until 15 days postoperatively on average (10).
A number of risk factors have found to be associated with SSI. Obesity is among the most commonly identified, with patients whose BMI is greater than 35 at 60% greater odds of postoperative infection (17,22). This association has been well established in different spine surgery techniques, with three principal underlying mechanisms. The first is that patients’ body habitus poses intraoperative challenges to exposure, requiring larger incisions, more extensive dissection, and as a result, longer operative times. Prolonged operative times are themselves independently associated with increased risk of infection, though this should not be interpreted causally given that it may serve as a proxy for case complexity. The second proposed mechanism is that obesity is a proxy for the thickness of subcutaneous tissue at the surgical site. Mehta et al. found that the ratio of fat thickness to total thickness from the lamina to the skin was independently associated with the risk of infection, with an odds ratio of 3.18. Furthermore, the average thickness of subcutaneous fat for patients who developed SSI was 5.6 mm greater in their study (23). The authors of the study propose that prolonged retraction leads to decreased blood flow and tissue necrosis, which in turn results in infection. The third mechanism is that comorbidities related to obesity, such as heart disease and diabetes, can impair wound healing. Interestingly, however, several studies have failed to identify a link between diabetes and SSI after PCF surgery (1,14,17), likely due to the inherent heterogeneity of the blood glucose control within patients with diabetes. Sebastian et al. also identified chronic steroid use as another modifiable risk factor for SSI, which is consistent with existing literature in other surgical fields that has found increased morbidity and mortality associated with their use (17). Patients taking steroids chronically are immunosuppressed, which impairs both wound healing and the immune system’s ability to fight pathogens. Patient with rheumatoid arthritis, for example, have been found to be at increased susceptibility for infection after PCF (22).
Strategies to prevent postoperative infection after PCF have largely employed the use of vancomycin powder applied intraoperatively to the wound. Strom et al. found that doing so significantly decreased the 1-year incidence of SSI from 10.9% to 2.5%, a finding corroborated by another recent study (20,22). However, another retrospective, single-center study found no significant reduction in infection rates after the addition of intrawound vancomycin powder, before or after adjusted analysis (18). Pahys et al. describe a series of prophylactic interventions that has resulted in a SSI rate to zero over 195 consecutive cases (22). First, the surgical site and drapes surround it are prepared with alcohol foam before standard preparation. Second, a superficial drain was placed in patients whose subcutaneous fat was estimated to exceed 2 cm. These two interventions resulted in a SSI rate of 0.3% over 323 cases. Subsequently, the addition of intrawound vancomycin prior to closure resulted in a SSI rate of zero over 195 cases. The other important strategy to reduce SSI in PCF is the meticulous closure of the wound in a multi-layered fashion, which reduces dead space and eliminating potential nidus for infection.
C5 palsy and other post-operative neurologic deficits
New postoperative neurologic deficits following PCF was found to occur in about 8.5% of patients (24). The most common deficit is C5 palsy, which manifests as weakness of the deltoid and/or biceps brachii muscles, with or without concomitant shoulder pain and sensory deficits. Even though about 96% of patients with minor palsies and 71% with severe palsies fully recover eventually, this is a dreaded complication due to its significant impact to the patient’s quality of life, and its highly variable time to recovery. One study has estimated time to recovery ranging from 48 hours to 41 months (25).
After the immediate postoperative period, the adverse impact of C5 palsy is observed on both a population level, where it is associated with increased resource utilization, and on an individual level, where quality of life and economic stability are affected. Miller et al. report that the development of postoperative C5 palsy is associated with an overall increased cost of $1,918 per patient (26). This is primarily accrued via the additional physical and occupational therapy sessions required. They found no significant differences in costs associated with hospital stays, surgery, or medications. The impact on hospital stays is considered to be minor, with an average prolongation of 1–2 days compared to patients who underwent uncomplicated PCF (11). The effect of C5 palsy on quality of life is significant, with patients reporting a significantly impaired capacity for self-care (measured on the EuroQol survey as ability to wash or dress oneself) in the short and long term (26).
C5 palsy affects between 6.7% and 9.5% of patients following PCF, with a recent systematic review by Pan et al. finding an average of 7.8% incidence across 28 studies (25,27,28). The etiology of this complication is not clear, but it has historically been attributed to iatrogenic injury, spinal cord ischemia and subsequent reperfusion injury, or tethering of the nerve from shifting of the spinal cord (29). The development of C5 palsy has been associated with greater than 5-fold odds of in-hospital mortality and greater than 2-fold odds of morbidity (24).
Few risk factors for the development of C5 palsy after PCF have been identified. Preoperative intervertebral foraminal stenosis is among the most consistently reported, with preop diameter less than 2.2 mm and postop diameter less than 2.3 mm associated with a 3-fold increase in risk (27,30). One proposed mechanism is that chronic preoperative compression of the C5 spinal nerve may be exacerbated by excessive reduction of anterolisthesis and the posterior shift of the spinal cord; however, recent evidence has argued against the contributions of spinal cord float back theory (27,31-33). Foraminotomy has been suggested as a measure to reduce C5 palsy rate (34-36), but it may not complete eliminate C5 palsy (37). Likewise, selective blocking laminoplasty to reduce spinal cord drift has been shown to be associated with reduced incidence of C5 palsy within 1 year of surgery (38).
Other risk factors for development of C5 palsy include advanced age and ossification of the posterior longitudinal ligament (OPLL), the latter of which is postulated to cause tethering on the nerve root (30,39). Of note, increased laminectomy width, which has been theorized to increase risk of C5 palsy by allowing greater spinal cord drift, has not been shown in recent studies to be an independent predictor (27,29).
Adjacent segment degeneration and junctional kyphosis
Among long-term complications following PCF, adjacent segment pathology, along with both proximal junctional kyphosis (PJK) and distal junctional kyphosis (DJK) are the most commonly identified and challenging to manage. Adjacent segment degeneration at the vertebral level next to the fusion construct may be radiographic only or clinically symptomatic. Associated radiographic findings include decreased intervertebral disc height or other degeneration, presence of osteophytes, and anterior ossification (40). Whether this process is attributable to increased mechanical stress and segmental motion resulting from vertebral fusion or simply part of the natural history of cervical spine degeneration remains a topic of controversy, but it is generally accepted that there is increased mechanical stress at the adjacent levels after fusion (41). Following PCF surgery, the incidence of new, radiologically diagnosed adjacent segment pathology is estimated to be 3.4% at 1 year and 5.9% at 2 years, with a further 11.8% of patients showing mild progression of previously observed spinal degeneration at adjacent vertebral levels (40,42). Long-term incidence has been estimated to be as high as 20–30% (40,41,43).
Junctional kyphosis is radiographically defined as a kyphotic deformity of either one level superior to the upper instrumented vertebra (UIV) in the case of PJK, or of the two levels inferior to the lowest instrumented vertebra (LIV) in DJK (Figure 2). There is not a clear consensus as to the degree of deformity necessary for diagnosis, with most studies establishing a threshold of 5 to 10 degrees of kyphosis as measured by the sagittal Cobb angle (44). Both PJK and DJK are postulated to have multifactorial etiologies, with contributions from poor bone quality, paraspinal muscle and interspinous ligament dissection, and instrumentation failure among others (44,45). Given that ligamentous and muscle dissection is a theorized contributor, PCF has been associated with higher rates of incidence compared to anterior cervical procedures, with rates ranging from 6% to 41% for PJK and up to 25% for DJK (44-46).
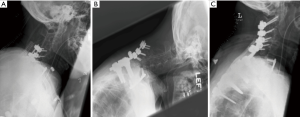
For both adjacent segment degeneration and junctional kyphosis, design of the fusion construct is crucial to preventing degeneration over the long term. The primary features to consider are the levels of the UIV and LIV. Proximally, much debate has centered on whether fusion should extend to C2 to bolster the fusion construct’s biomechanical strength or stop at C3 in order to allow for improved range of motion and reduce the amount of muscle dissection required (42,47). Though a recent study by Xia et al. found that fusion to C2 reduces the rate of proximal adjacent segment pathology from 5.0% to 0%, others have shown that placement of C2 pedicle screws are a major risk factor for PJK (42,44). Distally, whether to extend fusion to the upper thoracic spine remains controversial. The transition from the mobile, lordotic cervical spine to the rigid thoracic spine is subject to significant mechanical stress, and it has been theorized that stopping fusion at C7 allows for insufficient support at the distal end of the construct to withstand these forces (43). Some studies have shown significant reductions in adjacent segment pathology without impacting operative time or morbidity simply by extending fusion to T1, including a recent meta-analysis by Goyal et al. that found 2.75 times greater odds of fusion and fewer than half the rate of reoperations (43,48). Nonetheless, an edict for routinely including the thoracic spine in arthrodesis has not been established (49). The length of the fusion construct itself has not consistently been found to associate with the incidence of adjacent segment pathology or junctional kyphosis (42-45,49).
Pseudoarthrosis
Pseudoarthrosis is the failure of arthrodesis following a procedure intended to achieve fusion of the joint, known as a “false joint”. Failure of fusion is found in up to 20% of PCF patients, which likely represents an underestimation of the actual incidence given that as many as 30% are asymptomatic, and as many as 60% of revisions are related as well (15,20,50,51). Pseudoarthrosis is most commonly observed at the caudal end of fusion constructs, likely as a result of higher mechanical stress at the graft-body interface (51). Patients typically present with neck pain provoked by motion with possible radicular symptoms. Workup largely consists of imaging, which evaluates for a lack of bridging trabeculae between host bone and graft or excess motion, though pseudoarthrosis can be difficult to diagnose without surgical exploration.
Risk factors for pseudoarthrosis generally fall under two main categories: (I) impaired bone healing, or (II) increasing biomechanical stress on the fusion construct. Impaired bone healing may occur due to poor blood flow to the graft, as smoking and hypertension both are associated with 20% greater odds of pseudoarthrosis. Patients with a stunted inflammatory response, as observed in rheumatoid arthritis or chronic steroid use, have a 1.5–2.5 times greater risk of pseudoarthrosis as well (15). Increased biomechanical stress is largely a function of fusion length. Involvement of 4 to 8, and at least 9 vertebral segments were both associated with increased pseudoarthrosis rates, with odds ratios of 1.7 and 5.8 respectively (15). Interestingly, nonwhite race is a protective factor for unclear reasons.
Beyond management of underlying comorbidities, prevention strategies seek to maximize the strength of the construct while preventing undue force on focal areas of the fusion construct. As discussed above, incorporating vertebral segments T1–T4 into fusion have been found to increase strength and stability, partially due to the decreased range of motion afforded by more fusion levels and partially due to using pedicle screws at these levels (42,43). Accordingly, studies have shown a benefit of both extensions of fusion. Truumees et al. found that the rate of pseudoarthrosis among patients with LIV of T1 was 11.0%, nearly half that of patients with LIV of C7 at 21.2%, though clinical and radiographic outcomes were otherwise equivalent (50).
Incidental durotomy
Incidental durotomy is another complication can occur with posterior cervical spine surgeries (52). The incidence of dural tears is estimated to be between 0.8% and 3.9% (42,53,54), of which 7.0% to 32.2% of patients require postoperative treatment for persistent cerebrospinal fluid (CSF) drainage (55,56). Although positional headache, nausea and vomiting, neck pain, and changes in hearing are the most common presenting symptoms of CSF leaks, serious complications such as pseudomeningocele formation, nerve root entrapment, and intracranial hemorrhage rarely can occur (52). Among all cervical spine surgeries, older age, rheumatoid arthritis, longer operative time, more fusion levels, and poor preoperative neurologic status have been associated with increased risk of dural tear. However, revision surgery has been the only risk factor for incidental durotomy identified specifically with regards to posterior cervical surgeries (53).
Treatment modalities for dural tears include both intraoperative interventions such as direct suture repair, application of dural sealant and/or patch material, and postoperative interventions, including lumbar drain placement, bed rest, or revision surgical exploration and repair. To date, no single treatment strategy has been demonstrated to be superior, though intraoperative interventions are sufficient for about 90% of patients (55,57). Intraoperative dural sealant or patch use is the most commonly chosen strategy (53). This is often combined with direct suture repair of the defect, and lumbar drains can be placed as prophylaxis against CSF leaks as needed.
Discussion
Complications occur for all types of surgery, but the literature suggests that a posterior approach for cervical fusion has a 15% to 25% risk compared to less than 10% for ACDF. However, this observation should be interpreted cautiously for several reasons. First, PCF is generally a more appropriate choice for the older patient population with multilevel disease (typically >3 levels), and such patients often have more comorbidities and higher severity of spinal pathology with inherently increased risk for complication. Second, the vast majority of existing literature is retrospective in design, relying on either small, often single-center patient cohorts or large databases such as the NIS or National Surgical Quality Improvement Program (NSQIP) that do not have the granularity to define specific risk factors. This has the potential to limit the generalizability of adjusted analyses, as they may either lack the power to control for every variable of interest in the former case, or suffer from the absence of important covariates in the latter.
The most commonly observed complications following PCF are largely surgical complications, a result of the invasiveness of the procedure combined with the intricate anatomy and highly mobile nature of the cervical spine. In the acute to subacute postoperative phase, SSI and C5 palsy are of greatest concern. Both are associated with a significant increase in hospital resource utilization and impact patients’ quality of life. Dural tears are uncommonly observed intraoperatively, and the vast majority are closed primarily without requiring postoperative management. More chronically, degenerative spine conditions such as adjacent segment pathology and junctional kyphosis are observed because the fusion construct exerts mechanical forces on the adjacent cervical spine. Pseudoarthrosis is often a byproduct of these forces, most commonly found at the most caudal levels of the construct where the graft interfaces with the body’s normal bony architecture.
Risk factors found to be associated with the most common complications tended to put patients at increased risk by either increasing invasiveness or complexity of the procedure, impairing wound healing or increasing the stress resulting from the fusion construct. Patients who are obese, who require multi-level fusions, or those with severe manifestations of degenerative spine pathology all require greater intraoperative dissection to allow for proper instrumentation placement. Wound healing relies on both adequate blood flow and intact inflammatory response mechanisms, either of which can be impacted by patient comorbidities. Those with poor blood flow to the surgical site include patients who smoke and obese patients whose local tissues can necrose with prolonged retraction, whereas those with poor inflammatory responses include older or frailer patients, diabetics, and patients with autoimmune conditions on chronic steroids. Finally, fusion design has a significant impact on the risk of developing long term degeneration of the construct and adjacent vertebral levels. Biomechanical stress at the most cranial and caudal ends of fusion can lead to adjacent segment pathology and kyphosis, pseudoarthrosis, and reoperation.
Strategies to prevent complications have been identified at all stages of the perioperative period. Preoperatively, risk factor modification may be efficacious, and encouraging smoking cessation, stopping steroids when possible, prehabilitation programs to reduce frailty, and weight loss may mitigate risks. A thoughtful approach to choosing which levels to involve in arthrodesis is crucial as well, as some data shows that extension to C2 proximally and T1 distally provides additional support to prevent breakdown over time. Intra-operatively, taking extra steps to prevent infection such as additional skin preparation, drain placement, and intra-wound vancomycin has been demonstrated to reduce the incidence of SSI. Foraminotomy and selective blocking laminoplasties may reduce the risk of C5 palsy, although this must be balanced with the associated increase in blood loss and operative time (which has been associated with increased complications).
Conclusions
There are many potential complications associated with PCF. Clinicians should be aware of these complications and various prevention strategies to optimize clinical outcome in patients undergoing PCF.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lee A. Tan and Ilyas S. Aleem) for the series “Advanced Techniques in Complex Cervical Spine Surgery” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Dr Mummaneni is a consultant for DePuy Spine, Globus, and Stryker; has direct stock ownership in Spinicity/ISD; receives clinical/research support from NREF; recieves royalties from DePuy Spine, Thieme Publishers, and Springer Publishers; has a grant from AOSpine; and receives honoraria from Spineart. Dr. Chou is a consultant for Globus and Medtronic. Dr. Tan is a consultant for Stryker and Integrity Implants. The other authors have no conflicts of interest to declare.
Ethical Statement: The series “Advanced Techniques in Complex Cervical Spine Surgery” was commissioned by the editorial office without any funding or sponsorship. LAT serves as the unpaid editorial board member of Journal of Spine Surgery from Jan. 2019 to Jan. 2021 and served as the unpaid Guest Editor of the series. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Badiee RK, Chan AK, Rivera J, et al. Preoperative narcotic use, impaired ambulation status, and increased intraoperative blood loss are independent risk factors for complications following posterior cervical laminectomy and fusion surgery. Neurospine 2019;16:548-57. [Crossref] [PubMed]
- Medvedev G, Wang C, Cyriac M, et al. Complications, readmissions, and reoperations in posterior cervical fusion. Spine (Phila Pa 1976) 2016;41:1477-83. [Crossref] [PubMed]
- Campbell PG, Yadla S, Malone J, et al. Early complications related to approach in cervical spine surgery: single-center prospective study. World Neurosurg 2010;74:363-8. [Crossref] [PubMed]
- Memtsoudis SG, Hughes A, Ma Y, et al. Increased in-hospital complications after primary posterior versus primary anterior cervical fusion. Clin Orthop Relat Res 2011;469:649-57. [Crossref] [PubMed]
- Youssef JA, Heiner AD, Montgomery JR, et al. Outcomes of posterior cervical fusion and decompression: a systematic review and meta-analysis. Spine J 2019;19:1714-29. [Crossref] [PubMed]
- Badhiwala JH, Ellenbogen Y, Khan O, et al. Comparison of the inpatient complications and health care costs of anterior versus posterior cervical decompression and fusion in patients with multilevel degenerative cervical myelopathy: a retrospective propensity score-matched analysis. World Neurosurg 2020;134:e112-9. [Crossref] [PubMed]
- Tobin MK, Gragnaniello C, Sun FW, et al. Safety and efficacy of skipping C7 instrumentation in posterior cervicothoracic fusion. World Neurosurg 2019;130:e68-73. [Crossref] [PubMed]
- Highsmith JM, Dhall SS, Haid RW, et al. Treatment of cervical stenotic myelopathy: a cost and outcome comparison of laminoplasty versus laminectomy and lateral mass fusion. J Neurosurg Spine 2011;14:619-25. [Crossref] [PubMed]
- Shin JI, Kothari P, Phan K, et al. Frailty index as a predictor of adverse postoperative outcomes in patients undergoing cervical spinal fusion. Spine (Phila Pa 1976) 2017;42:304-10. [Crossref] [PubMed]
- DePasse JM, Durand W, Eltorai AEM, et al. Timing of complications following posterior cervical fusion. J Orthop 2018;15:522-6. [Crossref] [PubMed]
- Leckie S, Yoon ST, Isaacs R, et al. Perioperative complications of cervical spine surgery: analysis of a prospectively gathered database through the association for collaborative spinal research. Global Spine J 2016;6:640-9. [Crossref] [PubMed]
- Epstein NE. An argument for traditional posterior cervical fusion techniques: evidence from 35 cases. Surg Neurol 2008;70:45-51; discussion 51-2. [Crossref] [PubMed]
- Shamji MF, Cook C, Pietrobon R, et al. Impact of surgical approach on complications and resource utilization of cervical spine fusion: a nationwide perspective to the surgical treatment of diffuse cervical spondylosis. Spine J 2009;9:31-8. [Crossref] [PubMed]
- Worley N, Buza J, Jalai CM, et al. Diabetes as an independent predictor for extended length of hospital stay and increased adverse post-operative events in patients treated surgically for cervical spondylotic myelopathy. Int J Spine Surg 2017;11:10. [Crossref] [PubMed]
- Hofler RC, Swong K, Martin B, et al. Risk of pseudoarthrosis after spinal fusion: analysis from the healthcare cost and utilization project. World Neurosurg 2018;120:e194-202. [Crossref] [PubMed]
- Cheung JP, Luk KD. Complications of anterior and posterior cervical spine surgery. Asian Spine J 2016;10:385-400. [Crossref] [PubMed]
- Sebastian A, Huddleston P, Kakar S, et al. Risk factors for surgical site infection after posterior cervical spine surgery: an analysis of 5,441 patients from the ACS NSQIP 2005-2012. Spine J 2016;16:504-9. [Crossref] [PubMed]
- Martin JR, Adogwa O, Brown CR, et al. Experience with intrawound vancomycin powder for posterior cervical fusion surgery. J Neurosurg Spine 2015;22:26-33. [Crossref] [PubMed]
- Xu R, Bydon M, Sciubba DM, et al. Safety and efficacy of rhBMP2 in posterior cervical spinal fusion for subaxial degenerative spine disease: analysis of outcomes in 204 patients. Surg Neurol Int 2011;2:109. [Crossref] [PubMed]
- Strom RG, Pacione D, Kalhorn SP, et al. Decreased risk of wound infection after posterior cervical fusion with routine local application of vancomycin powder. Spine (Phila Pa 1976) 2013;38:991-4. [Crossref] [PubMed]
- Whitmore RG, Stephen J, Stein SC, et al. Patient comorbidities and complications after spinal surgery: a societal-based cost analysis. Spine (Phila Pa 1976) 2012;37:1065-71. [Crossref] [PubMed]
- Pahys JM, Pahys JR, Cho SK, et al. Methods to decrease postoperative infections following posterior cervical spine surgery. J Bone Joint Surg Am 2013;95:549-54. [Crossref] [PubMed]
- Mehta AI, Babu R, Sharma R, et al. Thickness of subcutaneous fat as a risk factor for infection in cervical spine fusion surgery. J Bone Joint Surg Am 2013;95:323-8. [Crossref] [PubMed]
- Dayananda S, Mehta A, Agarwal N, et al. Impact of perioperative neurologic deficits on clinical outcomes after posterior cervical fusion. World Neurosurg 2018;119:e250-61. [Crossref] [PubMed]
- Pan FM, Wang SJ, Ma B, et al. C5 nerve root palsy after posterior cervical spine surgery. J Orthop Surg (Hong Kong) 2017;25:2309499016684502. [Crossref] [PubMed]
- Miller JA, Lubelski D, Alvin MD, et al. C5 palsy after posterior cervical decompression and fusion: cost and quality-of-life implications. Spine J 2014;14:2854-60. [Crossref] [PubMed]
- Pennington Z, Lubelski D, Westbroek EM, et al. Spinal cord float back is not an independent predictor of postoperative C5 palsy in patients undergoing posterior cervical decompression. Spine J 2020;20:266-75. [Crossref] [PubMed]
- Nassr A, Eck JC, Ponnappan RK, et al. The incidence of C5 palsy after multilevel cervical decompression procedures: a review of 750 consecutive cases. Spine (Phila Pa 1976) 2012;37:174-8. [Crossref] [PubMed]
- Klement MR, Kleeman LT, Blizzard DJ, et al. C5 palsy after cervical laminectomy and fusion: does width of laminectomy matter? Spine J 2016;16:462-7. [Crossref] [PubMed]
- Nakashima H, Imagama S, Yukawa Y, et al. Multivariate analysis of C-5 palsy incidence after cervical posterior fusion with instrumentation. J Neurosurg Spine 2012;17:103-10. [Crossref] [PubMed]
- Heller JG, Silcox DH, Sutterlin CE. Complications of posterior cervical plating. Spine (Phila Pa 1976) 1995;20:2442-8. [Crossref] [PubMed]
- Tsuzuki N, Abe R, Saiki K, et al. Extradural tethering effect as one mechanism of radiculopathy complicating posterior decompression of the cervical spinal cord. Spine (Phila Pa 1976) 1996;21:203-11. [Crossref] [PubMed]
- Imagama S, Matsuyama Y, Yukawa Y, et al. C5 palsy after cervical laminoplasty: a multicentre study. J Bone Joint Surg Br 2010;92:393-400. [Crossref] [PubMed]
- Ohashi M, Yamazaki A, Watanabe K, et al. Two-year clinical and radiological outcomes of open-door cervical laminoplasty with prophylactic bilateral C4-C5 foraminotomy in a prospective study. Spine (Phila Pa 1976) 2014;39:721-7. [Crossref] [PubMed]
- Takemitsu M, Cheung KM, Wong YW, et al. C5 nerve root palsy after cervical laminoplasty and posterior fusion with instrumentation. J Spinal Disord Tech 2008;21:267-72. [Crossref] [PubMed]
- Liu T, Zou W, Han Y, et al. Correlative study of nerve root palsy and cervical posterior decompression laminectomy and internal fixation. Orthopedics 2010. [Crossref] [PubMed]
- Thompson SE, Smith ZA, Hsu WK, et al. C5 palsy after cervical spine surgery: a multicenter retrospective review of 59 cases. Global Spine J 2017;7:64S-70S. [Crossref] [PubMed]
- Kim GU, Lee GW. Selective blocking laminoplasty in cervical laminectomy and fusion to prevent postoperative C5 palsy. Spine J 2019;19:617-23. [Crossref] [PubMed]
- Chen Y, Chen D, Wang X, et al. C5 palsy after laminectomy and posterior cervical fixation for ossification of posterior longitudinal ligament. J Spinal Disord Tech 2007;20:533-5. [Crossref] [PubMed]
- Siemionow K, Monsef JB, Janusz P. Preliminary analysis of adjacent segment degeneration in patients treated with posterior cervical cages: 2-year follow-up. World Neurosurg 2016;89:730.e1-7. [Crossref] [PubMed]
- Lee JC, Lee SH, Peters C, et al. Risk-factor analysis of adjacent-segment pathology requiring surgery following anterior, posterior, fusion, and nonfusion cervical spine operations: survivorship analysis of 1358 patients. J Bone Joint Surg Am 2014;96:1761-7. [Crossref] [PubMed]
- Xia Y, Xu R, Kosztowski TA, et al. Reoperation for proximal adjacent segment pathology in posterior cervical fusion constructs that fuse to C2 vs C3. Neurosurgery 2019;85:E520-6. [Crossref] [PubMed]
- Schroeder GD, Kepler CK, Kurd MF, et al. Is it necessary to extend a multilevel posterior cervical decompression and fusion to the upper thoracic spine? Spine (Phila Pa 1976) 2016;41:1845-9. [Crossref] [PubMed]
- Lee J, Park YS. Proximal junctional kyphosis: diagnosis, pathogenesis, and treatment. Asian Spine J 2016;10:593-600. [Crossref] [PubMed]
- Passias PG, Vasquez-Montes D, Poorman GW, et al. Predictive model for distal junctional kyphosis after cervical deformity surgery. Spine J 2018;18:2187-94. [Crossref] [PubMed]
- Woods BI, Hohl J, Lee J, et al. Laminoplasty versus laminectomy and fusion for multilevel cervical spondylotic myelopathy. Clin Orthop Relat Res 2011;469:688-95. [Crossref] [PubMed]
- Jones EL, Heller JG, Silcox DH, et al. Cervical pedicle screws versus lateral mass screws. Anatomic feasibility and biomechanical comparison. Spine (Phila Pa 1976) 1997;22:977-82. [Crossref] [PubMed]
- Goyal A, Akhras A, Wahood W, et al. Should multilevel posterior cervical fusions involving c7 cross the cervicothoracic junction? A systematic review and meta-analysis. World Neurosurg 2019;127:588-95.e5. [Crossref] [PubMed]
- Lee DH, Cho JH, Jung JI, et al. Does stopping at C7 in long posterior cervical fusion accelerate the symptomatic breakdown of cervicothoracic junction? PLoS One 2019;14:e0217792. [Crossref] [PubMed]
- Truumees E, Singh D, Geck MJ, et al. Should long-segment cervical fusions be routinely carried into the thoracic spine? A multicenter analysis. Spine J 2018;18:782-7. [Crossref] [PubMed]
- Leven D, Cho SK. Pseudarthrosis of the cervical spine: risk factors, diagnosis and management. Asian Spine J 2016;10:776-86. [Crossref] [PubMed]
- Ishikura H, Ogihara S, Oka H, et al. Risk factors for incidental durotomy during posterior open spine surgery for degenerative diseases in adults: a multicenter observational study. PLoS One 2017;12:e0188038. [Crossref] [PubMed]
- O’Neill KR, Neuman BJ, Peters C, et al. Risk factors for dural tears in the cervical spine. Spine (Phila Pa 1976) 2014;39:E1015-20. [Crossref] [PubMed]
- Hannallah D, Lee J, Khan M, et al. Cerebrospinal fluid leaks following cervical spine surgery. J Bone Joint Surg Am 2008;90:1101-5. [Crossref] [PubMed]
- O’Neill KR, Fehlings MG, Mroz TE, et al. A multicenter study of the presentation, treatment, and outcomes of cervical dural tears. Global Spine J 2017;7:58S-63S. [Crossref] [PubMed]
- Baker GA, Cizik AM, Bransford RJ, et al. Risk factors for unintended durotomy during spine surgery: a multivariate analysis. Spine J 2012;12:121-6. [Crossref] [PubMed]
- Fang Z, Tian R, Jia YT, et al. Treatment of cerebrospinal fluid leak after spine surgery. Chin J Traumatol 2017;20:81-3. [Crossref] [PubMed]


