
Full endoscopic cervical spine surgery
Introduction
Cervical disc herniation is common and can affect patients of different ages (1). It can present as a radiculopathy or myelopathy, depending on the pattern of herniation. Cervical radiculopathy commonly presents with pain, paresthesia, and motor weakness (2). Cervical myelopathy can typically present with gait disturbance, motor weakness, loss of hand dexterity, bowel or bladder dysfunction, and paresthesia (3). Traditional surgical management includes anterior cervical discectomy and fusion (ACDF) and posterior cervical foraminotomy (PCF). These approaches have been exhaustively studied and validated (4-7). ACDF has long been considered the gold standard of cervical disc replacement techniques, but does pose some disadvantages, mainly the need for fusion and the risk of developed adjacent disc disease requiring future additional surgical intervention (8). With advances in endoscopic techniques and technologies, opportunities for development of minimally invasive and less disruptive surgical approaches presented itself.
Fully-endoscopic cervical spine surgery is different from micro-endoscopic cervical spine surgery in the sense that the tubular retractor is so small that even with loupes or microscope the operative field is not visible. Fully-endoscopic spine surgery is typically cervical spine surgery performed through a working-channel endoscope. Bi-portal fully endoscopic spine surgery, as the name implies, utilizes 2 ports: a port for surgical instruments and a port for an endoscopic camera. Endoscopic approaches to the cervical spine have been reported and found to be safe and effective in the literature (9).
Here we present 4 distinct fully endoscopic cervical spine surgery approaches for the treatment of cervical radiculopathy and myelopathy (with case series) that utilize a working channel endoscope, are truly minimally invasive, and do not require a fusion: (I) posterior cervical unilateral laminectomy and bilateral decompression, (II) PCF, (III) anterior cervical discectomy, and (IV) anterior transcorporeal discectomy.
Methods
Patient selection
A retrospective patient review was performed on 114 patients who underwent fully endoscopic cervical spine surgery procedures between 2012 and 2018. Minimum patient follow-up was 1 year.
Statistical analysis
Statistical calculations were carried out using IBM SPSS version 24 for Mac (IBM Corp.).
Posterior cervical unilateral laminectomy for bilateral decompression operative technique
The procedure was performed under general anesthesia; the patient was positioned prone on hip and chest bolsters with the head secured in the Mayfield head holder. Somatosensory evoked potential monitoring was used. The Joimax iLESSYS® Delta endoscopic system was used for the procedure. A 1 cm incision was made through the skin 1 cm lateral to the midline. Using intermittent fluoroscopic guidance, alternating between lateral and anterior-posterior (AP) view, a sequential dilators, then the final 10-mm beveled tubular retractor, were placed on the junction of the spinous process and the lamina. The 10-mm outer diameter working channel endoscope with 6 mm working channel was placed and a high speed Shrill® drill was used to complete the sub-spinous process laminectomy. An endoscopic micro Kerrison rongeur was used to remove the ligamentum flavum and to finish the laminectomy/laminotomy. Figure 1 demonstrates preoperative and postoperative MR images, intraoperative fluoroscopic images, and endoscopic camera views of a multi-level posterior cervical unilateral laminectomy for bilateral decompression.
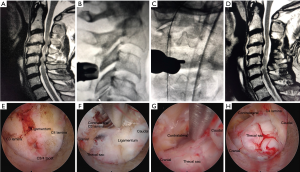
PCF operative technique
The procedure was performed under general anesthesia; patients were positioned prone on hip and chest bolsters with their heads secured in a Mayfield head holder. The Joimax iLESSYS® Pro endoscopic system with a 7.3 mm outer diameter was used for the procedure. Percutaneous entry was established entering through the skin 1 cm lateral to the midline. Using intermittent fluoroscopic guidance, alternating between lateral and AP view, a 3.5-inch 18-gauge needle was advanced, and the tip placed on junction of the inferior lamina and facet complex. A Kirschner wire (K-wire) was placed, then the needle removed, and sequential dilators and then the 7-mm beveled tubular retractor were placed on the junction of the superior part of the inferior lamina at the facet junction. The Shrill® diamond drill was used to perform the foraminotomy which was completed with the endoscopic micro Kerrison punch. The foraminotomy was completed when the exiting nerve root could be visualized, and the decompression was carried lateral to the inferior pedicle. Figure 2 demonstrates the preoperative images, intraoperative fluoroscopic images, and endoscopic camera views from an endoscopic PCF.
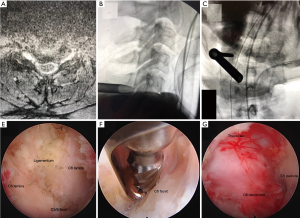
Anterior cervical discectomy operative technique
The procedure was performed under general anesthesia; patients were positioned supine. The Wolf Vertebris Cervical scope was used for the procedure. Lateral fluoroscopy was used to determine the level of the incision. A 5 mm incision was made just medial to the sternocleidomastoid muscle. Blunt dissection was done medial to the carotid and jugular and blunt dissection was used to mobilize the esophagus off the prevertebral space. The 1st blunt dilator was subsequently placed through the disc space under bi-planar fluoroscopy monitoring. After sequential dilation, the final tubular retractor was advanced through the disc space under fluoroscopic control. The working channel endoscope was advance and the Shrill® drill was used to drill down the uncinate joint to allow access for the endoscopic graspers to remove the disc herniation. Figure 3 demonstrates the preoperative images, intraoperative fluoroscopic images, and endoscopic camera views from an endoscopic anterior cervical discectomy.

Anterior cervical transcorporeal discectomy operative technique
The procedure was performed under general anesthesia; patients were positioned supine. The approach was similar to the anterior cervical discectomy technique. A 1.5 cm incision was made just medial to the sternocleidomastoid muscle, blunt finger dissection was done with a standard Smith-Robinson approach. A 13-mm Matrix tubular retractor was placed first over the upper vertebral body, a guide pin was placed into the upper vertebral body with planned trajectory toward the disc herniation. Next, a 6.5-mm cannulated power drill was used to drill over the guide pin, and a Shrill® drill was used to finish drilling a channel through the superior vertebra to allow placement of the tubular retractor with its distal opening at the junction of the inferior endplate of the superior vertebral body and the posterior longitudinal ligament (PLL). The TESSYS® (6.3 mm outer diameter) was used, and instead of entering the disc with the tubular retractor, drilling was performed using intermittent fluoroscopic guidance, alternating between lateral and AP view, through the superior vertebral body to safely target the disc pathology. The channel created allowed access to the herniated ventral disc fragment without violating the anterior portion of the disc. Figure 4 demonstrates a preoperative image, intraoperative fluoroscopic image, and endoscopic camera views from an endoscopic anterior cervical transcorporeal discectomy procedure.
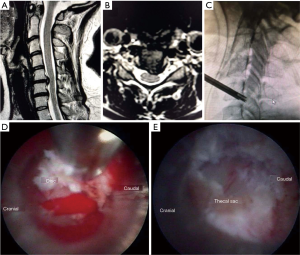
Results
Posterior cervical unilateral laminectomy for bilateral decompression operative technique
Twenty-one patients were in this case series with a mean age of 68 (range, 57–86) years. Mean preoperative modified Japanese Orthopaedic Association (mJOA) score was 10.2. The mean operation time was 58 minutes/level, and blood loss was minimal. There was no intraoperative or postoperative complication, including cord or nerve root injury, C5 nerve palsy, dural tear/cerebrospinal fluid (CSF) leak, hematoma, or infection. Mean hospital stay was 0.6 (range, 0–1) day.
The mean 1-year postoperative mJOA score was 15.6, recovery rates 52.9%, showing statistically significant improvement (P<0.001). Clinically, muscle weakness and sensory deficit significantly improved in all patients. Gait improved in 16 patients. No patient showed neurologic deterioration or increased kyphosis after surgery.
PCF operative technique
Sixty-eight patients were in this case series with a mean age of 56 (range, 32–86) years.
The mean operation time was 42 minutes/level and blood loss was minimal. There were no intraoperative complications, no postop hematoma or infection, and none of the patients required a revisional operation in the early post-operative period. Mean baseline visual analog scores (VAS) scores of neck (nVAS), VAS scores of arm (aVAS), and neck disability index (NDI) were 6.3 (4.0–10.0), 5.6 (3.0–8.0), and 41.6 (22.9–49.6), respectively. The 1-year follow-up values were: nVAS 1.6 (0.0–4.0), aVAS 1.2 (0.0–3.0), NDI 14.7 (4.6–16.8) (P<0.001, respectively).
Anterior cervical discectomy operative technique
Nineteen patients were in this case series with a mean age of 38 (range, 30–56) years. There were no intraoperative or postoperative complications. One patient developed recurrent herniation and underwent ACDF surgery within 3 months of index surgery. For the remaining 18 patients: nVAS for neck pain reduced from average 7.2 (range, 6–10) to 1.6 (range, 0–3); aVAS for arm pain reduced from 5.6 (range 3 to 9) to 1.2 (range 0 to 3) at 1-year follow up. NDI reduced from 58.7 (range 32.8 to 68.2) to 12.8 (range 8.2 to 16.9) at 1-year follow up, all statistically significant (P<0.02).
Anterior cervical transcorporeal discectomy operative technique
Six patients were in this case series with a mean age of 32 (range, 28–42) years. Estimated blood loss was minimal, no intraoperative or postoperative complication, no recurrent disc herniation (no patient required a subsequent ACDF). nVAS for neck pain reduced from average 7.6 (range, 6–10) to 0.8 (range, 0–2) at 1-year follow up. NDI reduced from 49.8 (range 36.8 to 72.6) to 10.6 (range 7.2 to 16.7) at 1-year follow up, all statistically significant (P<0.01).
Discussion
Our experiences with four distinct fully endoscopic cervical spine approaches reveal highly successful outcomes with effective reduction in symptoms, no significant complications, minimal blood loss, and short hospital stays. Our results are aligned with other author’s experiences with such techniques.
Indications for cervical endoscopic spine surgery were the same as those for traditional anterior and posterior techniques. Most patients in this study sought out care by the senior surgeon because they were interested in the most minimally invasive procedure possible.
When compared to other reports of anterior endoscopic approaches, our study cohort experienced similar rates of decrease in nVAS and aVAS scores and improvement in NDI scores. For instance, in a retrospective study of 187 patients undergoing anterior endoscopic cervical discectomy, Parihar et al. show decrease in VAS scores for arm and neck pain, 6.7 to 1.7 and 3.2 to 1.1, respectively, with only 2 patients requiring a second procedure with mean follow up of 29 months (10). This same series showed that despite lack of fusion and placement of an intervertebral spacer, cervical lordosis improved. However, reports in the literature have varied when it pertains to cervical lordosis, some suggesting that ACDF is superior in maintaining proper cervical alignment. Results have ranged from improved, unchanged, and worsened lordosis (11,12). However, these reports also show that clinical outcomes were improved despite the different cervical alignments seen on imaging (11,12). Similarly, Lee and Lee found that despite loss of disc height after fully anterior endoscopic cervical discectomy, their patients report improved nVAS and aVAS as well as NDI scores (13). Yet, with ACDF patients are at risk for injuries during dissection, may develop pseudarthroses, and may develop adjacent segment disease. All of these complications are mitigated with the use of endoscopic techniques (8,14,15). Long term data, however, remains scarce. Our experiences with anterior endoscopic discectomy techniques show similar resolution of symptoms and improvement in NDI and VAS scores as has been reported in the literature.
Similarly, successful results have been reported in posterior endoscopic techniques as well. A recent study by Wan et al., reports on their experience with posterior percutaneous full endoscopic cervical discectomy under local anesthesia (16). Twenty-five patients underwent the procedure with 24 achieving resolution of symptoms, and no major complications were reported (16). Another study looked at strength recovery after fully-endoscopic posterior cervical discectomy (FEPCD) and reported a 95% rate of strength recovery with 86% of full resolution of weakness at 1 year follow up (17). Similar rates of favorable outcomes (>85%) have been reported in the literature (18). Proponents of endoscopic posterior approaches have argued that post-operative pain is minimized due to the lack of muscle stripping, blood lose is avoided, operative time is reduced, and hospital stays are shortened. Our results are consistent with these beliefs and add to a growing body of favorable research into endoscopic approaches. However, a prospective large volume trial comparing ACDF or PCF with FEPCD has not been undertaken yet. Such a trial will help better delineate the role of endoscopic approaches as compared with traditional cervical approaches in the treatment of cervical radiculopathies and myelopathies.
Fully endoscopic cervical spine surgery is an emerging minimally invasive surgical option for the treatment of symptomatic cervical radiculopathy and myelopathy. Advantages include the high definition visualization of the surgical pathology, the reduction of tissue trauma, and taking advantage of familiar anatomical approaches. Anterior cervical endoscopic discectomy techniques offer additional advantages including preservation of movement at that level and avoiding fusion and minimal to no manipulation of nerve roots. Main disadvantages include the steep learning curve of endoscopic techniques for surgeons accustomed to traditional open approaches, the limited direct field of view, and the narrow working channel. However, multiple strategies can be used to mitigate these downsides. First, surgeons may take advantage of multiple cadaver course to accelerate their progress on the learning curve. Moreover, surgeons may start with more simple endoscopic procedures to familiarize themselves with the approach. Flexible instruments have helped overcome the limitations of a narrow channel, and device development has been rapid.
In brief, we present 4 illustrative case series from a high-volume endoscopic spine surgery center in the United States that highlight the similar outcomes of endoscopic cervical spine surgery to those of traditional cervical spine surgery. The results reported regarding the effectiveness and success of these techniques are similar to what has already been reported in the literature and add to the growing cases of successfully fully endoscopic cervical spine surgery. Endoscopic cervical approaches offer a novel and promising treatment option for cervical radiculopathy and myelopathy. However, our experience remains limited by its retrospective nature, the lack of a direct comparison arm, limited follow-up data, and a relatively small patient population. Larger long-term prospective trials with a control arm are still needed to tease out the true potential and shortcomings of endoscopic approaches to the cervical spine as compared to traditional techniques.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Hisashi Koga and Alf Giese) for the series “Full-endoscopic Spine Surgery” published in Journal of Spine Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jss.2019.10.15). The series “Full-endoscopic Spine Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by Lifespan IRB; Project Title: (600415-8) Endoscopic Spine Surgery; Principal Investigator: Albert Telfeian, MD.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Woods BI, Hilibrand AS. Cervical radiculopathy: epidemiology, etiology, diagnosis, and treatment. J Spinal Disord Tech 2015;28:E251-9. [Crossref] [PubMed]
- Rhee JM, Yoon T, Riew KD. Cervical radiculopathy. J Am Acad Orthop Surg 2007;15:486-94. [Crossref] [PubMed]
- Iyer A, Azad TD, Tharin S. Cervical spondylotic myelopathy. Clin Spine Surg 2016;29:408-14. [Crossref] [PubMed]
- Martins AN. Anterior cervical discectomy with and without interbody bone graft. J Neurosurg 1976;44:290-5. [Crossref] [PubMed]
- Rosenørn J, Hansen EB, Rosenørn MA. Anterior cervical discectomy with and without fusion. A prospective study. J Neurosurg 1983;59:252-5. [Crossref] [PubMed]
- Bohlman HH, Emery SE, Goodfellow DB, et al. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am 1993;75:1298-307. [Crossref] [PubMed]
- Villavicencio AT, Pushchak E, Burneikiene S, et al. The safety of instrumented outpatient anterior cervical discectomy and fusion. Spine J 2007;7:148-53. [Crossref] [PubMed]
- Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976) 2007;32:2310-7. [Crossref] [PubMed]
- Yang JS, Chu L, Chen L, et al. Anterior or posterior approach of full-endoscopic cervical discectomy for cervical intervertebral disc herniation? A comparative cohort study. Spine (Phila Pa 1976) 2014;39:1743-50. [Crossref] [PubMed]
- Parihar VS, Yadav N, Ratre S, et al. Endoscopic anterior approach for cervical disc disease (disc preserving surgery). World Neurosurg 2018;115:e599-609. [Crossref] [PubMed]
- Haden N, Latimer M, Seeley HM, et al. Loss of inter-vertebral disc height after anterior cervical discectomy. Br J Neurosurg 2005;19:469-74. [Crossref] [PubMed]
- Kim CH, Shin KH, Chung CK, et al. Changes in cervical sagittal alignment after single-level posterior percutaneous endoscopic cervical diskectomy. Global Spine J 2015;5:31-8. [Crossref] [PubMed]
- Lee JH, Lee SH. Clinical and radiographic changes after percutaneous endoscopic cervical discectomy: a long-term follow-up. Photomed Laser Surg 2014;32:663-8. [Crossref] [PubMed]
- Kelly MP, Eliasberg CD, Riley MS, et al. Reoperation and complications after anterior cervical discectomy and fusion and cervical disc arthroplasty: a study of 52,395 cases. Eur Spine J 2018;27:1432-9. [Crossref] [PubMed]
- Wang MC, Chan L, Maiman DJ, et al. Complications and mortality associated with cervical spine surgery for degenerative disease in the United States. Spine (Phila Pa 1976) 2007;32:342-7. [Crossref] [PubMed]
- Wan Q, Zhang D, Li S, et al. Posterior percutaneous full-endoscopic cervical discectomy under local anesthesia for cervical radiculopathy due to soft-disc herniation: a preliminary clinical study. J Neurosurg Spine 2018;29:351-7. [Crossref] [PubMed]
- Lee U, Kim CH, Chung CK, et al. The recovery of motor strength after posterior percutaneous endoscopic cervical foraminotomy and discectomy. World Neurosurg 2018;115:e532-8. [Crossref] [PubMed]
- Yang B, Xie J, Yin B, et al. Treatment of cervical disc herniation through percutaneous minimally invasive techniques. Eur Spine J 2014;23:382-8. [Crossref] [PubMed]


