
Early surgical intervention among patients with acute central cord syndrome is not associated with higher mortality and morbidity
Introduction
Acute central cord syndrome (ACS) constitutes nearly 44% of all traumatic spinal cord injury (SCI), which makes it the most common form of incomplete SCI (1,2). However, the influence of surgical timing in ACS is controversial and recent reports have shown conflicting results regarding mortality in this patient population.
ACS typically occurs in older patients after a cervical hyperextension injury—especially in the presence of pre-existing stenosis of the spinal canal (2,3). The current literature generally supports surgical decompression of the spinal cord after SCI as a means of attenuating the development of secondary injury processes and improving neurological outcomes (3-7). Recent studies have indicated the benefit of early decompression for improved neurological outcomes (3,8). While the recommendations for early intervention remain debated, some authors have reported increased mortality in the early surgical cohort (<24 hours) across 1,060 patients in a National Trauma Data Bank (NTDB) (1). This data could potentially advocate for delaying surgery in the acute setting for ACS to allow for medical optimization and possible reduction in mortality.
However, ACS remains difficult to study and in many cases is limited to observational or large-scale administrative data bases. To date cohort studies investigating the impact of surgical timing on neurological outcomes and survival in patients with ACS have yielded variable results (1,2,9,10). While randomized controlled trials (RCT) are considered the clinical gold standard for evaluating treatment effect, in many situations ethical and logistic constraints render this study design impractical. Because of the limited sample sizes of these cohort studies, it has been difficult to arrive at definitive and reproducible conclusions (1,2,9,10). Similarly, even with large administrative database studies, several confounding variables often exist between the early and late surgery groups such as injury severity, comorbidities, and age. Propensity score matching (PSM) methods could allow one to mimic some of the characteristics of RCT in the setting of an observational cohort study by evaluating the probability of treatment assignment (surgical timing) conditional on observed baseline covariates [injury severity, Charlson comorbidity index (CCI), age, et cetera] (11).
In this study we sought to determine if early surgical intervention for acute traumatic central cord was associated with increased all-cause mortality among patients with multisystem trauma in the NTDB. Due to the fact that observational studies of choice of treatment and surgical timing may have limited validity due to selection bias and confounding factors, we performed a propensity analysis to adjust for possible confounders that may have contributed to previous findings of increased mortality in early surgical intervention for ACS (1,11).
We hypothesized that following propensity matching, early surgical decompression would not be associated with greater mortality.
Methods
The NTDB is the largest prospective national trauma database with more than 900 contributing trauma centers and other hospitals nationwide. It is supported by the American College of Surgeons and serves as data saturated tool for healthcare providers and researchers.
Study population
Using the NTDB from years 2011 to 2014, all patients over the age of 18 with acute traumatic central cord syndrome (ATCCS), as identified using International Classification of Diseases, Ninth Revision (ICD-9) codes for central spinal cord injuries (Table S1), were included.
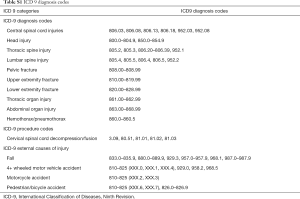
Full table
Clinical data
Data on baseline demographics (age, gender), trauma center level (level 1 to 5), medical risk factors, injury severity, surgical intervention, hospital course, and in-patient all-cause mortality were collected from the NTDB.
Comorbidities included alcoholism, cancer, congestive heart failure, coronary artery disease, dementia, diabetes mellitus, functionally dependent status, hypertension, liver disease, obesity, peripheral vascular disease, renal disease, respiratory disease, and prior stroke with neurological deficits. For a global description of comorbidities, the modified CCI was utilized in statistical models (12).
Injury characteristics included head, thoracic, lumbar, pelvic and extremity injuries (Table S1). The injury severity score (ISS), was abstracted from the NTDB (7). The ISS correlates with mortality, morbidity, and hospitalization time after trauma; values of 15 or above indicate multisystem trauma. Additional injury characteristics included Glasgow Coma Scale (GCS) on arrival and the presence of alcohol and drugs.
Hospital course data including time to surgical intervention, minor adverse events, significant adverse events, and all-cause in-hospital mortality were abstracted from the NTDB. The primary end point was all-cause mortality. Surgical intervention was determined by ICD-9 procedure code (Table S1). Time to surgery was categorized as early surgery if less than 24 hours and late surgery if more than 24 hours. Serious adverse events (SAE) included acute respiratory distress syndrome, cardiac arrest requiring cardiopulmonary resuscitation, myocardial infarction, severe sepsis, stroke, thromboembolic event, or an unplanned return to the operating room (1).
Statistical analysis
Our cohort is described using means ± standard deviation and counts (%) as appropriate. Differences between patients who died were compared to those that survived, and those who underwent early versus late surgery were compared using chi-square statistics for categorical variables and t-tests for continuous variables. Univariate regression analyses including the sole predictor of early surgery and multivariate regression analyses adjusted for covariates were performed to determine predictors of mortality and mortality + SAE. Because timing of surgery was not assigned at random, a propensity score for early surgery was developed to account for any potential selection and confounding biases. The methods underlying PSM has been previously described (11). Our propensity model predicted the probability of early surgery from the following covariates: patient age, gender, ISS, CCI, GCS total score, alcohol and drugs present, head injury, and hospital ACS level. The propensity score ranged from 0.13 to 0.59, representing the likelihood that a patient would undergo early surgery. We then used propensity scores to match each early surgery patient to control patients that underwent late surgery using nearest neighbor matching. Control cases were not constrained to be used once and the matched analyses were weighted on control case use. Regression models were then repeated using the matched sample. Age, ISS, and CCI were used as covariates in adjusted regression models to replicate previous research (1). Statistical significance was set at a threshold of P<0.05. All analyses with exception of matching were performed using SPSS version 22 (SPSS, Inc, Chicago, IL, USA) Matching was performed using STAT. A version 14.2 (StataCorp LP, College Station, TX, USA).
Results
Patient characteristics
The sample consisted of 2,379 patients, 731 (30.7%) of whom underwent early surgery for ACS. The mean age in the sample was 56.3±15.2 years and 1,886 (79.3%) were male. Baseline demographic and clinical data are summarized in Table 1. Patients undergoing early surgery for ACS were more likely to be White (P=0.042), younger (P<0.001), have a lower CCI (P<0.001), a higher ISS (P=0.040), and require intensive care unit (ICU) hospitalization (P<0.001). The prevalence of the following comorbidities was significantly lower for the early surgery group: hypertension (P=0.001), alcoholism (P=0.004), diabetes (P=0.001), respiratory disease (P=0.022), and cardiac disease (P=0.011).
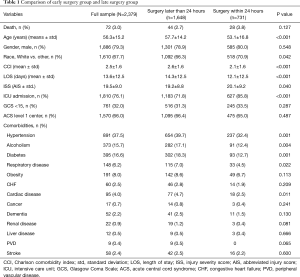
Full table
Early surgery and mortality
During hospitalization for ACS following trauma, 72 (3.0%) died. There was not a significant association between early surgery and mortality (3.8% vs. 2.7%, P=0.127). Mortality outcomes based on surgical timing are reported in Table 2. Within both the early and late surgery groups, inpatient mortality was associated with older mean age (P=0.001, P<0.001, respectively), higher mean CCI (P=0.001, P<0.001), and higher mean ISS (P=0.009, P=0.007). In the early surgery cohort, inpatient mortality was associated with a higher proportion of patients with an admission GCS <15 (P=0.007). Within the late surgery cohort, a higher proportion of the mortalities utilized the ICU in comparison to non-mortalities (P<0.001).
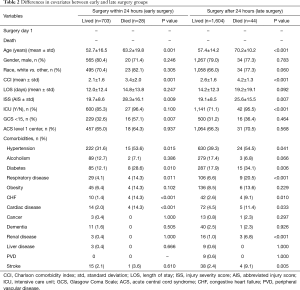
Full table
Covariate analysis
Comparisons between the early and late surgery group for predictors of the propensity model are shown in Table 3 using the full sample and the propensity-matched sample. Age and CCI were highly significant in the full sample with high P values of 0.907 and 0.895 in the matched sample indicating the match was effective. The presence of alcohol was the sole nonsignificant covariate in the full sample that was significant in the matched sample, suggesting the match was not effective. However, from a clinical standpoint, the prevalence was within 5% for the early and late surgery groups (28.0% vs. 23.3%) and considered unlikely to impact results.
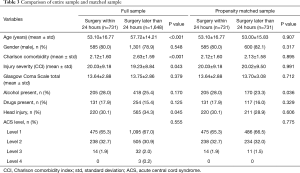
Full table
Regression analyses
In unadjusted models, regardless of sampling, early surgery was not predictive of mortality or SAE + mortality (Table 4). In models adjusted for age, ISS, and CCI, early surgery was predictive of mortality and SAE + mortality using the full sample only. Significance values for early surgery predicting mortality increased from a significant 0.013 in the full model to nonsignificant values of 0.107 in the matched sample. Similarly, early surgery was predictive of SAE + mortality in the full model (P=0.027) but not in the matched (P=0.255) model.
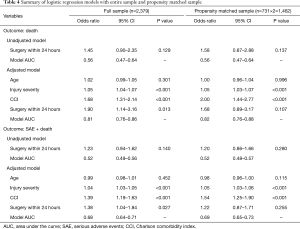
Full table
Discussion
Several previous studies have compared surgical versus medical management for ACS and have produced a consensus in support of surgical treatment in terms of American Spinal Injury Association (ASIA) motor scores at discharge and return of neurological function (8,13-15). However, the timing between injury and surgery remains controversial and there is a paucity of strong statistical analysis attempting to tackle this problem. While the risk of early intervention is not well defined, some authors have suggested a higher likelihood of death associated with surgical intervention independent of injury severity (1). The goal of the current study was to perform a more sophisticated statistical analysis using propensity scores to better delineate the association of early surgical intervention with mortality in patients suffering from ACS in a NTDB.
Using the NTDB, we identified a total of 2,379 patients with ACS following trauma. Of these patients, a total of 731 (30.7%) underwent surgery for ACS within 24 hours. In several previous publications, early surgery was consistently associated with poor outcome using unadjusted (un-matched models). However, the association of early surgery was eliminated in the matched models. This suggests the presence of unadjusted confounders in traditional statistical models, something that matching helps account for. Likely, this stems from the fact that the early surgery group demonstrated significantly higher ISS, which was found to be a significant predictor of death in this group.
Timing following CCS remains controversial. A meta-analysis in 2017 by Wilson et al. (16) of 449 relevant citations found significant variability in terms of the effect of early surgical intervention (≤24 hours) on patient performance on subsequent neurological testing. However, there is a general consensus that early surgery is safe and perhaps even superior in terms of neurological recovery following ACS. Thus, despite continuing investigation, early surgical intervention for ACS is considered the current standard of practice (17,18). For instance, Fehlings et al. found improved neurologic outcome as measured by ASIA motor scores in their early surgical intervention group (≤24 hours after injury) compared to their late surgical intervention group (>24 hours after injury) (17). Similarly, Chen et al. found that earlier surgery translated to improved outcomes at 6 months and final follow-up compared to the delayed surgery group (14). Our findings corroborate these data demonstrating the safety and efficacy of early intervention in ACS and use a large patient group and statistical matching to attempt to mitigate some of the aforementioned controversy.
Despite this, in a study of 211 patients with ACS, Aarabi et al. (19) showed no significant relationship between time before surgery and ASIA motor score, or Functional Independence Score (FIM). Additionally, a recent study by Samuel et al. (1), which similarly used NTDB data, reported an association between delayed surgical intervention and a decreased odds of inpatient mortality. This translated to a 19% decrease in odds of mortality with each 24-hour increase in time until surgery (1). This is in contrast to a recent meta-analysis by Anderson et al. that used 5 large databases to conclude that surgery for ACS in less than 24 hours appeared safe and effective (8). If early intervention in fact was neutral or detrimental to patient morbidity and mortality compared to delayed intervention, this may call into question the aforementioned standard of care regarding ACS. However, although Samuel et al. took into account the severity of patient injury via the ISS and CCI, they assumed average values for these numbers when calculating the reduction in overall risk of mortality (1). Our study, which utilized a more nuanced matching technique, emerged with a different outcome.
Propensity-matching is a statistical technique commonly used to adjust for confounders in observational studies that extends beyond traditional multivariate analysis. Yet it begs the question, is this advanced matching necessary? In this paper, we can conclude that matching appears to provide a benefit beyond simply adjusting for the sample size. There was no difference in the significance of any of the covariates or early surgery from the matched sample. However, there is the question as to which is the correct model. Authors in the past have commented “all models are wrong, but some are useful” (20). Are we more correct to report the results using full sample size along with the potential for confounding or to address potential confounding and report results from an adjusted data set? Based on our analysis, it suggests that there are hidden confounders that may sway the results of previous studies to favor later intervention. Based on our data, these confounders, if adequately controlled, result in contrary conclusions that match previous clinical reports and consensus statements (9,11,15,16).
Limitations
The most significant limitation of this study is the lack of randomization for surgical timing assignment. The use of observational studies for investigation of treatment effects remains controversial. However, study design and statistical analyses may negate or effectively reduce the associated bias. In this study, we used propensity analysis in an attempt to enable a more rigorous adjustment for selection bias and confounding factors than would be possible with standard multivariate analyses. Additionally, it is important to consider the inherent limitations of administrative, multicenter registries such as the NTDB when interpreting the results of this study such as treatment protocol heterogeneity, patient factors, and data entry. Notably, the NTDB only tracks in-patient morality; we were unable to evaluate 30-day all-cause mortality associated with the procedure or hospitalization. Furthermore, the available data lack granularity regarding details of the neurological injury, such as the level of injury, neurological status (ASIA impairment grade, weakness, etc.), and neurological outcomes, all of which could impact surgical decision-making and affect choice of early vs late intervention. However, despite lack of granularity, such data provides valuable, high volume insight into a relatively uncommon and unpredictable pathology such as cervical SCI and central cord syndrome.
Conclusions
There does not appear to be an association between early surgical intervention and increased mortality in the setting of acute central cord syndrome. We theorize that using the NTDB to analyze survival is confounded by patient factors including existing comorbidities and multisystem trauma, rather than timing of surgical intervention. Delaying definitive surgical care may predispose patients to worsened disposition and greater neurological morbidity.
Acknowledgments
None.
Footnote
Conflicts of Interest: Dr. JS Uribe: stock shareholder, grant/research support recipient, and consultant for NuVasive, Inc. as well as consultant for SI-Bone and Misonix. Dr. JD Turner: consultant for SeaSpine and NuVasive, Inc. grant/research support recipient. A portion of the manuscript was presented at the 2017 AANS/CNS Joint Section on Disorders of the Spine and Peripheral Nerves Las Vegas, Nevada, on March 8-11, 2017. Abstract title: Early Surgical Intervention for Acute Central Cord Syndrome is Not Associated with Increased Mortality. Abstract authors: Jakub Godzik, MD; Jay Dalton; Rohit Mauria, BS; Alan Cook; Kristina Chapple, PhD; Jay D. Turner, MD, PhD.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Institutional Review Board approval was not required for this retrospective database review study. No participants’ informed consent was necessary for our study.
References
- Samuel AM, Grant RA, Bohl DD, et al. Delayed surgery after acute traumatic central cord syndrome is associated with reduced mortality. Spine (Phila Pa 1976) 2015;40:349-56. [Crossref] [PubMed]
- Yoshihara H, Yoneoka D. Trends in the treatment for traumatic central cord syndrome without bone injury in the United States from 2000 to 2009. J Trauma Acute Care Surg 2013;75:453-8. [Crossref] [PubMed]
- Fehlings MG, Vaccaro A, Wilson JR, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS One 2012;7:e32037. [Crossref] [PubMed]
- Carlson GD, Gorden CD, Oliff HS, et al. Sustained spinal cord compression: part I: time-dependent effect on long-term pathophysiology. J Bone Joint Surg Am 2003;85:86-94. [Crossref] [PubMed]
- Delamarter RB, Sherman J, Carr JB. Pathophysiology of spinal cord injury. Recovery after immediate and delayed decompression. J Bone Joint Surg Am 1995;77:1042-9. [Crossref] [PubMed]
- Guha A, Tator CH, Endrenyi L, et al. Decompression of the spinal cord improves recovery after acute experimental spinal cord compression injury. Paraplegia 1987;25:324-39. [PubMed]
- Dimar JR 2nd, Glassman SD, Raque GH, et al. The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine (Phila Pa 1976) 1999;24:1623-33. [Crossref] [PubMed]
- Anderson KK, Tetreault L, Shamji MF, et al. Optimal Timing of Surgical Decompression for Acute Traumatic Central Cord Syndrome: A Systematic Review of the Literature. Neurosurgery 2015;77 Suppl 4:S15-32. [Crossref] [PubMed]
- Anderson DG, Sayadipour A, Limthongkul W, et al. Traumatic central cord syndrome: neurologic recovery after surgical management. Am J Orthop (Belle Mead NJ) 2012;41:E104-8. [PubMed]
- Cadotte DW, Fehlings MG., et al. Spinal cord injury: a systematic review of current treatment options. Clin Orthop Relat Res 2011;469:732-41. [Crossref] [PubMed]
- Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol 1999;150:327-33. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Bose B, Northrup BE, Osterholm JL, et al. Reanalysis of central cervical cord injury management. Neurosurgery 1984;15:367-72. [Crossref] [PubMed]
- Chen TY, Dickman CA, Eleraky M, et al. The role of decompression for acute incomplete cervical spinal cord injury in cervical spondylosis. Spine (Phila Pa 1976) 1998;23:2398-403. [Crossref] [PubMed]
- Chen TY, Lee ST, Lui TN, et al. Efficacy of surgical treatment in traumatic central cord syndrome. Surg Neurol 1997;48:435-40; discussion 441. [Crossref] [PubMed]
- Wilson JR, Tetreault LA, Kwon BK, et al. Timing of Decompression in Patients With Acute Spinal Cord Injury: A Systematic Review. Global Spine J 2017;7:95S-115S. [Crossref] [PubMed]
- Fehlings MG, Tetreault LA, Wilson JR, et al. A Clinical Practice Guideline for the Management of Patients With Acute Spinal Cord Injury and Central Cord Syndrome: Recommendations on the Timing (≤24 Hours Versus >24 Hours) of Decompressive Surgery. Global Spine J 2017;7:195S-202S. [Crossref] [PubMed]
- La Rosa G, Conti A, Cardali S, et al. Does early decompression improve neurological outcome of spinal cord injured patients? Appraisal of the literature using a meta-analytical approach. Spinal Cord 2004;42:503-12. [Crossref] [PubMed]
- Aarabi B, Hadley MN, Dhall SS, et al. Management of acute traumatic central cord syndrome (ATCCS). Neurosurgery 2013;72 Suppl 2:195-204. [Crossref] [PubMed]
- Box GE. Science and Statistics. J Am Stat Assoc 1976;71:791-9. [Crossref]


