
Comparison of M6-C and Mobi-C cervical total disc replacement for cervical degenerative disc disease in adults
Introduction
In recent years cervical total disc replacement (CTDR) has emerged as an effective alternative to anterior cervical discectomy and fusion (ACDF) for the treatment of cervical degenerative disc diseases (1). Fusion however is an alteration to the natural biomechanics of the spine, which immobilises the spinal motion segment and increases intradiscal pressure at adjacent segments (2). As such, spinal fusion is postulated to accelerate degeneration of the adjacent spinal segments through these increased stresses (2-4), and deterioration of adjacent vertebral levels may eventually culminate in the development of further pathology, so called, adjacent segment degeneration (ASD) (5).
Due to these drawbacks, many surgeons are trending towards so called “motion-sparing technology” such as CTDR, if clinically appropriate. This technology, through the preservation of segmental range of motion (ROM) and alleviation of intradiscal pressures, may theoretically minimise the risk of ASD (6,7). However, one of the recognised complications of CTDR is heterotopic ossification (HO). HO is defined as abnormal bone growth outside of the skeletal system, and, in the context of CTDR, HO refers to growth of bone around the implanted disc replacement device. This bone growth can restrict the ROM of the operative spinal segments, and potentially negate any ASD-sparing advantages that CTDR may have over ACDF.
The exact mechanism behind the development of HO after CTDR remains elusive. One theory is that changes in biomechanical factors of the spinal segment after CTDR may stimulate the development of ectopic bone (8). HO may be a self-defence mechanism to immobilise the spinal segment to prevent non-physiological motion after CTDR (8). Given the non-physiological motion of spinal segments after CTDR using current prostheses, a next-generation prosthesis, M6-C prosthesis (Spinal Kinetics, Sunnyvale, California, USA), has been developed to better replicate the natural movement of intervertebral disc (9). Theoretically, the M6-C can better simulate kinematics of human intervertebral disc than that of a constrained ball-and-socket–type prosthesis design (10,11).
The present study aims to: (I) compare clinical and radiological outcomes between the M6-C and Mobi-C prostheses (12), (II) compare the rate of HO between M6-C and Mobi-C prostheses, and (III) explore the associations between the changes in biomechanical factors and the rate of HO. Both M6-C and Mobi-C (LDR Medical, Troyes, France) are two commonly used prostheses in CTDR (13). We hypothesized that the incidence and grades of HO would be significantly lower in patients who received M6-C prosthesis than those who received Mobi-C prosthesis in short-term follow-up.
Methods
This study is a retrospective analysis of prospectively recorded data. Medical records were reviewed including basic demographics, radiological images and clinical parameters, of 114 patients who were treated with CTDR between C2 to C7 spinal levels by a single senior spine surgeon (14) at a single institution in Sydney, Australia.
Patients who underwent CTDR between March 2004 and April 2017 and met the inclusion and exclusion criteria were included in the study. Ethics approval was acquired through the local health district research committee (HREC ref no: 17/060). Surgical indication was symptomatic radiculopathy and/or myelopathy secondary to cervical degenerative disc disease, which did not respond to conservative treatments for at least three months. There was no restriction to the number of cervical levels operated. Types of implants used during the trial period included M6-C, Mobi-C and Prestige LP (Medtronic Sofamor Danek, Memphis, TN, USA) cervical disc prostheses. The type of prosthesis used was based on the preference of patients and the surgeon based on a preoperative discussion, implants approved at the time, and factors of the individual patient. Three types of CTDR operations were included: single-level, multilevel CTDR, and hybrid surgery (combined CTDR and ACDF).
Patients with less than 1-year radiological follow-up and those that did not develop HO within the year of follow-up were excluded. Other exclusion criteria are shown in Table 1. Inclusion and exclusion criteria were examined by reviewing medical records, pre-operative radiographs, CT and MRI images.
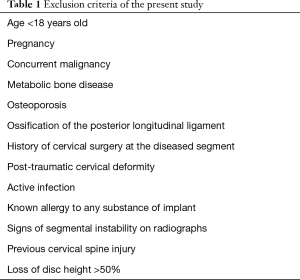
Full table
Applying the above criteria, we excluded 52 patients who had less than 1-year radiological follow-up and who did not develop HO, resulting in a total of 62 subjects and 63 surgeries (1 patient had a 2-level CTDR at other cervical levels after the first CTDR).
Next-generation prosthesis
In light of the incompatibility between conventional cervical disc replacement prostheses and spine kinematics of human, M6-C prosthesis has been designed to structurally and functionally replicate that of the natural intervertebral disc (15).
The core of the M6-C prosthesis is composed of (I) an inner compressible nucleus core, and (II) an outer annulus made of high tensile strength fibre, which can limit segmental ROM within physiological parameters by applying progressive resistance (16). The elastic properties of the artificial nucleus and annulus allow the M6-C to rotate and translate in every possible axes and planes (anterior-posterior direction, side to side, and axial compression) (16). The disc core is further encapsulated by a polymer sheath, which avoids tissue in-growth and migration of wear debris. The endplates have three titanium-coated keels on the surfaces, which can increase bone-contact surface area and achieve immediate press-fit implant stability (16).
Surgical approach
Following general anaesthesia, a standard right-sided anterior approach to the cervical spine, through the anterior triangle was performed. This involves a horizontal linear incision at the appropriate level with dissection along the medial aspect of the sternocleidomastoid muscle, to identify the musculo-visceral column. The oesophagus and trachea were medially retracted while the carotid sheath was laterally retracted to expose the anterior vertebral surface in the midline. A Caspar distractor (A-Spine ASIA, Taiwan) was used to distract the disc space at the operative level and a discectomy was performed to remove the degenerated intervertebral disc and ensure complete decompression of neurological structures. After preparation of the implant bed, a template prosthesis was trialled to confirm sizing before the appropriate device was inserted and the wound closed. The patient was required to wear a soft collar and use anti-inflammatory medication for two weeks postoperatively.
A number of strategies based on the evidence in the literature have been adopted by the surgeon to minimise the risk of HO in our study: (I) copious irrigation of the operative site with normal saline (17-19); (II) maximise end-plate coverage (20); (III) minimise bleeding of damaged bone/Caspar hole bleeding by using SurgiFlow (Johnson & Johnson, USA) or bone wax (21); and (IV) minimal cauterisation and careful dissection of the longus colli muscle (22,23). Since the evidence on the efficacy of non-steroidal anti-inflammatory drugs (NSAIDs) for HO prevention is inconsistent, we did not routinely administer NSAIDs as a prophylaxis of HO before the operation (24).
Clinical evaluation
Basic demographics included age, gender, ethnicity, body mass index (BMI), tobacco use, and surgical indication.
Patient-reported clinical parameters included the following:
- Visual Analog Scale Score (VAS), which assesses the severity and frequency of pain of the body in general.
- Neck Disability Index (NDI), which assesses the impact of neck pain on patients’ daily functions and activities.
- Medical Outcomes Study 12-Short Form (SF-12) Mental Component Summary (MCS), which assesses mental health status of patients.
- SF-12 Physical Component Summary (PCS), which assesses physical health status of patients.
All of the clinical outcomes were collected by a research assistant, with no bias as to patient outcomes.
Radiological evaluation
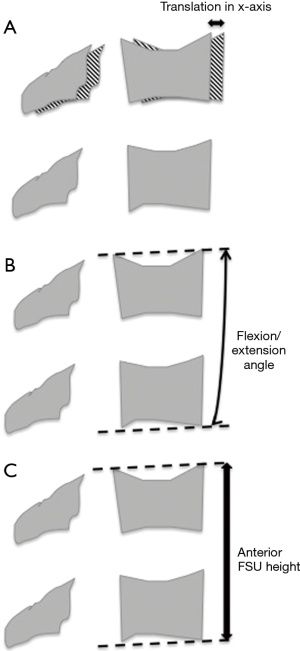
Biomechanical measurements were based on lateral and/or dynamic flexion/extension views of standing radiographs and CT scan images, conducted on a quantitative motion analysis software (Figure 1). Segmental and cervical flexion/extension ROM were assessed by the Cobb method (25). We measured the Cobb angle formed between the endplates of rostral and caudal vertebral to determine the functional spinal unit (FSU) flexion and extension angles. We also measured the Cobb angle formed between the inferior endplates of C2 and C7 vertebrae to determine the global cervical flexion and extension angles. A lordotic angle was defined to be a positive value. Migration of the device was defined as translation of the prosthesis by >2 mm in the antero-posterior plane (26). Translation in other axes was not measured in our study.
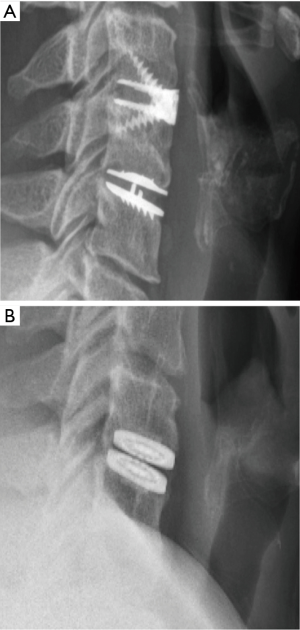
Radiological images were also reviewed to establish the diagnosis and grading of HO. The McAfee grading system was adopted to gauge HO from grade 0 to grade IV, based on either X-ray or CT images (Figure 2) (27). Patients were not required to undergo any additional tests or procedures as a result of the study.
Statistical analysis
Data on continuous variables were presented in mean (± standard deviation) while categorical variables were presented in number (percentage). Independent student t-test and Fisher’s exact tests were used to compare continuous and categorical variables between M6-C and Mobi-C groups respectively. If the continuous variable was not normally distributed, between-group difference would be analysed by Mann-Whitney U test. Dependent student t-test was used to assess changes in clinical and radiological outcomes from baseline to final follow-up. Also, Fisher’s Exact Test was adopted to identify categorical variables that might be related to the development of HO. Finally, sub-group analysis was performed based on the types of prosthesis. Statistical significance was defined as P<0.05. All statistical analysis was conducted on SPSS version 25.0 (IBM Corporation, Armonk, New York).
Results
Baseline demographics
Of the 62 included subjects, 63 surgeries were performed, in which 34 were single-level CTDR, 27 were two-level procedures and 2 were three-level procedures. Among the 94 spinal segments operated, 73 segments received CTDR while 21 received ACDF (Redmond, A-Spine ASIA, Taiwan). The most commonly operated level was C5/C6 (42.9%), followed by C6/C7 (28.6%). The mean radiological follow-up was 29.0 months (3–84 months), which includes 7 patients who had less than 12 months follow-up and developed HO. Table 2 summarises the baseline characteristics of subjects included in our study.
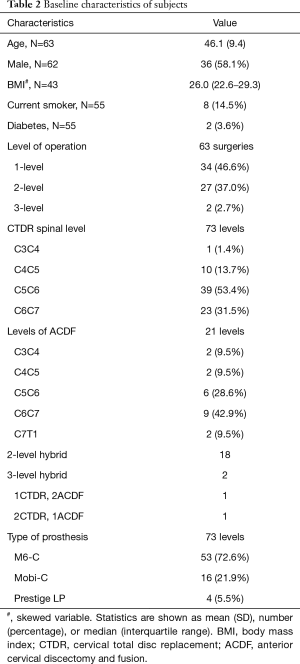
Full table
Clinical outcomes
The mean clinical follow-up was 36.9 months (8–82 months). Table 3 shows the clinical outcome measures at baseline and final follow-up. SF-12 total score (P=0.001), SF-12 PCS (P<0.0001) and VAS pain (P<0.0001) were significantly improved at final follow-up with respect to their pre-operative baseline. SF-12 PCS significantly increased from 37.4 (±8.5) pre-operation to 46.9 (±8.8). VAS pain significantly decreased from 6.4 (±2.1) pre-operation to 3.2 (±2.3). On the other hand, NDI, SF-12 MCS did not significantly change (P>0.05) at final follow-up compared to baseline.

Full table
Radiological outcomes
Table 4 summarises the radiological outcomes at baseline and final follow-up. Flexion-extension X-ray revealed that neither FSU ROM nor cervical ROM changed at final-follow-up. Based on neutral radiological images, there were also no significant changes in anterior and posterior FSU height at final follow-up.

Full table
The overall incidence of HO at final follow-up was 70.4% (50/71 surgical spinal segments). Table 5 provides the distribution of grades of HO. Thirteen surgical spinal segments developed grade I HO; 19 had grade II HO; 13 had grade III HO; and 5 had grade IV HO. Twenty-one spinal segments did not develop HO at their final follow-up.

Full table
Two spinal segments migrated anteriorly 3 mm or more, which met the criteria of device migration (Table 6) (26). However, none of these spinal segments had device failure or needed re-operation.

Full table
Comparison between M6-C and Mobi-C
M6-C group comprised of 44 subjects, in which there were 29 male and 15 female. Mobi-C group composed of 15 subjects, in which 6 were male and 9 were female. There were no statistical differences in demographics and baseline characteristics of subjects between the M6-C and Mobi-C groups, including age, gender, BMI, smoking history, the type of surgery, ROM and FSU height (Table 7).
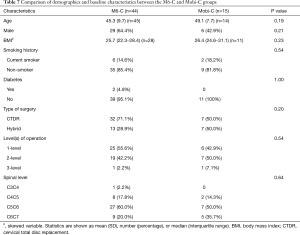
Full table
M6-C group demonstrated significant improvements in the NDI and SF-12 PCS scores from baseline to final follow-up whereas CTDR using Mobi-C prosthesis did not significantly improve these outcomes (Table 8). In M6-C group, NDI decreased from 42.8% (±20.3) pre-treatment to 14.0% (±10.6). SF-12 PCS increased from 36.7 (±9.6) pre-treatment to 46.1 (±9.8). Both groups significantly improved SF-12 total and VAS pain scores. However, for all the clinical outcome measures, there were no statistically significant differences in clinical outcomes at final follow-up between the M6-C and Mobi-C groups. The changes in clinical outcomes from baseline to final follow-up were also comparable between the two groups.
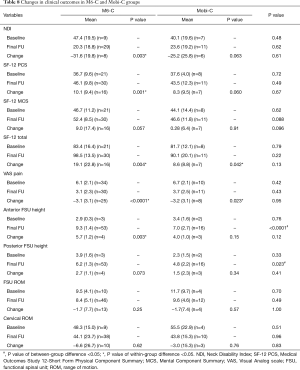
Full table
In regards to radiological outcomes, anterior and posterior FSU height were significantly higher in M6-C group than that in Mobi-C group at final follow-up, but not FSU and cervical ROM. However, the changes in all radiological outcomes were not significantly different between the two groups. Based on X-ray and CT images, 37 out of 52 spinal segments (71.2%) developed HO in M6-C group while 10 out of 16 spinal segments developed HO in Mobi-C group (62.5%). The rate of HO in M6-C group was comparable with that in Mobi-C group (relative risk: 1.14, 95% confidence interval: 0.75–1.73). The associations of the changes in radiological outcomes with the rate of HO were not significant (Table 9).

Full table
Discussion
CTDR was developed to preserve ROM of operative spinal motion segments, to reduce adjacent segment intradiscal pressure and minimise the risk of developing ASD. However, the development of HO may limit segmental ROM at the implanted level, which is against the primary purpose of CTDR. In theory, M6-C structurally and functionally closer replicates the motion of the natural intervertebral disc than that of constrained ball-and-socket–type prosthesis design (10,11). Our study is the first, to our best knowledge, to compare the rate of HO between a new-generation prosthesis (M6-C) and Mobi-C. Both the M6-C and Mobi-C are two commonly used prostheses in CTDR (13), in the Australian health system.
In terms of functional outcomes, the M6-C significantly improved multiple outcome measures, including NDI, SF-12 PCS, SF-12 total and VAS pain scores, but not SF-12 MCS score. Although the Mobi-C group did not significantly improve NDI and SF-12 PCS scores, there were no differences in any functional outcome measures between the M6-C and Mobi-C groups. The insignificant differences between the groups suggest that the M6-C can restore functional impairments to the same degree to that of Mobi-C prosthesis in short-term follow-up. Our results are in accordance with the two published articles on the M6-C prosthesis, which showed that CTDR with M6-C prosthesis significantly improved various functional outcomes of patients 1 to 2 years after CTDR (9,16).
The causes of HO are largely unknown. A number of theories have been proposed by scholars including: residual bone dust in the disc space (17-19), implant-endplate mismatch (20), trauma to the endplate for preparation to fit the prosthesis (21), and trauma to the longus colli muscle (22,23). These theories have heralded corresponding surgical measures to minimise the rate of HO. Although we have adopted these measures recommended in the literature to minimise the risk of HO, the overall incidence of HO in our study seemed to be higher than that of other studies. Reyes-Sanchez recruited 36 patients who had CTDR with M6-C prosthesis and no HO was found at 2-year follow-up (16). Moreover, a meta-analysis conducted by Chen and colleagues reported the overall prevalence of HO to range from 44.6% to 58.3% after 1–2 years follow-up (28), while another recent meta-analysis reported the HO rate after 1–2 years follow-up to be 38% (29). No cervical prosthesis to date can avert the development of HO. Given the positive association between the length of follow-up and the rate of HO (29), the wide range of follow-up period in our study might have explained the higher rate of HO.
Prior to the development of HO, there seems to be a “window period” that the prosthesis still maintains its functions and preserves segment ROM. It has been reported that there is a linear association between the length of follow-up and the rate of HO (29). Theoretically, the temporary preservation of segmental ROM may delay the formation of ASD. A recent meta-analysis demonstrated that CTDR was superior to ACDF in reducing adjacent segment disease in the short-term (30). Although no study has explored the association between follow-up period and severity of HO grade, we hypothesize that the progression of HO is dependent on time. Given enough time, all patients may eventually develop HO after CTDR, with eventual fusion of the operative segment.
Despite the theoretical advantages of M6-C prosthesis in restoring biomechanical functions of spine, the results of the present study did not support this theory in the clinical setting. In short-term radiological follow-up, anterior and posterior FSU height was significantly higher in M6-C group than that of Mobi-C group at final follow-up. However, the changes in radiological parameters, including FSU height, were comparable between the two groups. Hence, M6-C prosthesis restored biomechanics of spine to the same degree to that of Mobi-C prosthesis. In contrast to our insignificant results, Pham and colleagues reported that the overall range of extension of Mobi-C was significantly higher than that of M6-C at 3-month follow-up (13). However, this result is limited by the brief period of follow-up.
Our study has only measured FSU height and ROM, and cervical ROM. There is a number of kinematics parameter of the spinal segment the present study did not address, such as centre of rotation and translation in the y-axis. We cannot exclude the possibility that M6-C is superior to current prostheses in restoring spinal kinematics that were not measured in the present study.
The present study has a number of limitations that merit consideration. Firstly, the small sample size reduces the power of our study. Second, although recent clinical trials supported higher sensitivity and specificity of CT scan in detecting HO over plain radiographs (31,32), CT scans were not routinely used in the current study. This is due to the concern of cost-effectiveness and unnecessary exposure to higher doses of radiation if CT scan was not indicated. Therefore, the use of plain radiographs in our study may underestimate the rate and grade of HO. Last but not least, the single-surgeon, single-institution design of our study and the comparatively young cohort of patients may further compromise the external validity of the study. Local factors can influence results of a single-institution study and it is difficult to adjust for selection bias. Hence, our results may be limited to a particular geographic location and may not be generalizable to the national population.
Conclusions
Patients who underwent CTDR due to refractory cervical degenerative disc disease demonstrated significant improvements in some clinical outcome measures in comparison with baseline values, but not improvements in radiological parameters. When stratified by the type of prosthesis, the changes in functional and radiological outcomes were comparable between M6-C and Mobi-C prosthesis. The rate of HO was comparable between the two groups. Changes in radiological outcomes were not associated with the rate of HO. Thus, our study showed that CTDR with M6-C prosthesis was as effective as Mobi-C in restoring clinical and radiological outcomes of patients in short-term follow-up. Studies with longer follow-up period are needed to discern whether CTDR using M6-C prosthesis will reduce the rate of HO and ASD in comparison with conventional prostheses.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethics approval was acquired through the local health district research committee (HREC ref no: 17/060).
References
- Coric D, Kim PK, Clemente JD, et al. Prospective randomized study of cervical arthroplasty and anterior cervical discectomy and fusion with long-term follow-up: results in 74 patients from a single site. J Neurosurg Spine 2013;18:36-42. [Crossref] [PubMed]
- Eck JC, Humphreys SC, Lim TH, et al. Biomechanical study on the effect of cervical spine fusion on adjacent-level intradiscal pressure and segmental motion. Spine (Phila Pa 1976) 2002;27:2431-4. [Crossref] [PubMed]
- Dmitriev AE, Kuklo TR, Lehman RA Jr, et al. Stabilizing potential of anterior, posterior, and circumferential fixation for multilevel cervical arthrodesis: an in vitro human cadaveric study of the operative and adjacent segment kinematics. Spine (Phila Pa 1976) 2007;32:E188-96. [Crossref] [PubMed]
- Cunningham BW, Hu N, Zorn CM, et al. Biomechanical comparison of single-and two-level cervical arthroplasty versus arthrodesis: effect on adjacent-level spinal kinematics. Spine J 2010;10:341-9. [Crossref] [PubMed]
- Matsumoto M, Okada E, Ichihara D, et al. Anterior cervical decompression and fusion accelerates adjacent segment degeneration: comparison with asymptomatic volunteers in a ten-year magnetic resonance imaging follow-up study. Spine (Phila Pa 1976) 2010;35:36-43. [Crossref] [PubMed]
- Chang UK, Kim DH, Lee MC, et al. Range of motion change after cervical arthroplasty with ProDisc-C and prestige artificial discs compared with anterior cervical discectomy and fusion. J Neurosurg Spine 2007;7:40-6. [Crossref] [PubMed]
- Heller JG, Sasso RC, Papadopoulos SM, et al. Comparison of BRYAN cervical disc arthroplasty with anterior cervical decompression and fusion: clinical and radiographic results of a randomized, controlled, clinical trial. Spine (Phila Pa 1976) 2009;34:101-7. [Crossref] [PubMed]
- Cho HJ, Shin MH, Huh JW, et al. Heterotopic ossification following cervical total disc replacement: iatrogenic or constitutional? Korean J Spine 2012;9:209-14. [Crossref] [PubMed]
- Thomas S, Willems K, Van den Daelen L, et al. The M6-C cervical disk prosthesis: first clinical experience in 33 patients. Clin Spine Surg 2016;29:E182-7. [Crossref] [PubMed]
- Phillips FM, Garfin SR. Cervical disc replacement. Spine (Phila Pa 1976) 2005;30:S27-33. [Crossref] [PubMed]
- Sekhon LH, Ball J. Artificial cervical disc replacement: principles, types and techniques. Neurol India 2005;53:445-50. [Crossref] [PubMed]
- HPS2-Thrive Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203-12. [Crossref] [PubMed]
- Pham M, Phan K, Teng I, et al. Comparative Study Between M6-C and Mobi-C Cervical Artificial Disc Replacement: Biomechanical Outcomes and Comparison with Normative Data. Orthop Surg 2018;10:84-8. [Crossref] [PubMed]
- Nieuwdorp M, Vergeer M, Bisoendial R, et al. Reconstituted HDL infusion restores endothelial function in patients with type 2 diabetes mellitus. Diabetologia 2008;51:1081-4. [Crossref] [PubMed]
- Patwardhan A, Tzermiadianos M, Tsitsopoulos P, et al. Primary and coupled motions after cervical total disc replacement using a compressible six-degree-of-freedom prosthesis. Eur Spine J 2012;21:618-29. [Crossref] [PubMed]
- Reyes-Sanchez A, Miramontes V, Olivarez LMR, et al. Initial clinical experience with a next-generation artificial disc for the treatment of symptomatic degenerative cervical radiculopathy. SAS J 2010;4:9-15. [Crossref] [PubMed]
- Leung C, Casey AT, Goffin J, et al. Clinical significance of heterotopic ossification in cervical disc replacement: a prospective multicenter clinical trial. Neurosurgery 2005;57:759-63. [Crossref] [PubMed]
- Wenger M, van Hoonacker P, Zachee B, et al. Bryan cervical disc prostheses: preservation of function over time. J Clin Neurosci 2009;16:220-5. [Crossref] [PubMed]
- Yi S, Shin DA, Kim KN, et al. The predisposing factors for the heterotopic ossification after cervical artificial disc replacement. Spine J 2013;13:1048-54. [Crossref] [PubMed]
- Thaler M, Hartmann S, Gstöttner M, et al. Footprint mismatch in total cervical disc arthroplasty. Eur Spine J 2013;22:759-65. [Crossref] [PubMed]
- Shichang L, Yueming S, Limin L, et al. Clinical and radiologic comparison of dynamic cervical implant arthroplasty and cervical total disc replacement for single-level cervical degenerative disc disease. J Clin Neurosci 2016;27:102-9. [Crossref] [PubMed]
- Tu TH, Wu JC, Huang WC, et al. Heterotopic ossification after cervical total disc replacement: determination by CT and effects on clinical outcomes. J Neurosurg Spine 2011;14:457-65. [Crossref] [PubMed]
- GUéRIN P, Obeid I, Bourghli A, et al. Heterotopic ossification after cervical disc replacement: clinical significance and radiographic analysis. A prospective study. Acta Orthopædica Belgica 2012;78:80-6. [PubMed]
- Tu TH, Wu JC, Huang WC, et al. Postoperative nonsteroidal antiinflammatory drugs and the prevention of heterotopic ossification after cervical arthroplasty: analysis using CT and a minimum 2-year follow-up. J Neurosurg Spine 2015;22:447-53. [Crossref] [PubMed]
- Cobb J. Outline for the study of scoliosis: instructional course lecture 5. Edwards. Ann Arbor, MI 1948.
- Gercek E, Arlet V, Delisle J, et al. Subsidence of stand-alone cervical cages in anterior interbody fusion: warning. Eur Spine J 2003;12:513-6. [Crossref] [PubMed]
- McAfee PC, Cunningham BW, Devine J, et al. Classification of heterotopic ossification (HO) in artificial disk replacement. J Spinal Disord Tech 2003;16:384-9. [PubMed]
- Chen J, Wang X, Bai W, et al. Prevalence of heterotopic ossification after cervical total disc arthroplasty: a meta-analysis. Eur Spine J 2012;21:674-80. [Crossref] [PubMed]
- Kong L, Ma Q, Meng F, et al. The prevalence of heterotopic ossification among patients after cervical artificial disc replacement: a systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e7163. [Crossref] [PubMed]
- Zhu Y, Zhang B, Liu H, et al. Cervical disc arthroplasty versus anterior cervical discectomy and fusion for incidence of symptomatic adjacent segment disease: a meta-analysis of prospective randomized controlled trials. Spine (Phila Pa 1976) 2016;41:1493-502. [Crossref] [PubMed]
- Tu TH, Wu JC, Huang WC, et al. Postoperative nonsteroidal antiinflammatory drugs and the prevention of heterotopic ossification after cervical arthroplasty: analysis using CT and a minimum 2-year follow-up. J Neurosurg Spine 2015;22:447-53. [Crossref] [PubMed]
- Brenke C, Scharf J, Schmieder K, et al. High prevalence of heterotopic ossification after cervical disc arthroplasty: outcome and intraoperative findings following explantation of 22 cervical disc prostheses. J Neurosurg Spine 2012;17:141-6. [Crossref] [PubMed]


