
Computer-assisted surgical navigation is associated with an increased risk of neurological complications: a review of 67,264 posterolateral lumbar fusion cases
Introduction
Neurologic injuries from iatrogenic pedicle wall breaches during screw placement are known complications of posterolateral lumbar fusions (PLF) with an estimated risk of 0.8% to 6.1% (1-3). To minimize the risk of these complications, computer-assisted surgical navigation and/or intraoperative neuromonitoring (ION) are often used. Somatosensory evoked potentials (SSEPs) and motor-evoked potentials (MEPs) monitor spinal cord function while electromyography (EMG) is a form of ION that monitors peripheral muscle activity from nerve root stimulation due to pedicle screw malposition. On the other hand, computer-assisted navigation surgery (NAV) involves the use of a 3-dimensional computed-tomography or fluoroscopy-based navigational guidance for pedicle screw placement.
While ION and NAV have been shown to decrease the risk of neurological injury in spinal deformity surgery, their use in routine PLF remains controversial (4-15). Proponents of the routine use of ION or NAV for PLF claim that it improves both the accuracy and safety of pedicle screw implantation while opponents refute this claim by citing increased cost and resource utilization, increased surgical time, and no improvement in patient outcomes with ION or NAV. To date, there is a dearth of literature directly comparing ION and NAV in PLFs. As such, the goal of this study was to: (I) evaluate the trends in the use of ION and/or NAV for instrumented PLFs in the United States; and (II) assess the risk of complications (including neurological injuries) and reoperation for pedicle screw revision within 90 days of surgery following PLF with and without ION and/or NAV.
Methods
Retrospective longitudinal analyses were performed using the Truven Health MarketScan® databases from 2007–2015. This large, nationally-representative resource includes de-identified data on over 149 million unique patients during this time frame from more than 100 large employers, managed care organizations, hospitals, EMR providers, Medicare, and Medicaid. The data contains unique patient identifiers allowing longitudinal patient tracking for assessment of patient comorbidities and outcomes.
Data collection
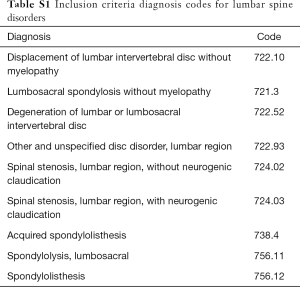
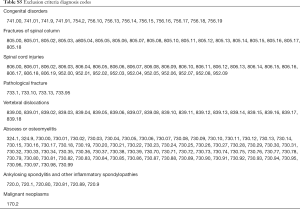
Inpatient, primary lumbar fusions with posterior instrumentation for diagnoses of disc herniation, lumbosacral spondylosis, disc degeneration, spinal stenosis, spondylolisthesis, and/or spondylolysis were identified using the ICD-9-CM and CPT codes (see Tables S1-S6). Patients under the age of 18 and revision surgeries were excluded, as were diagnoses of pregnancy, neoplasms, intraspinal abscesses, osteomyelitis, discitis, fracture, dislocation, vehicular accidents, inflammatory spondyloarthropathies, or rheumatoid arthritis. Patients with any of the following concomitant procedures were also excluded: interbody fusion, application of biomechanical device, thoracic posterolateral fusion, osteotomies, and corpectomies. The latter three exclusion criteria eliminated spinal deformity surgeries and the interbody exclusion kept the analysis specific to PLFs only.
Full table

Full table
Full table

Full table
Full table

Full table
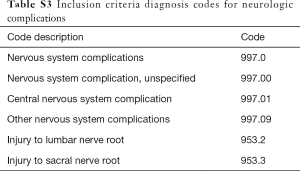
NAV and ION were identified with CPT codes (see Tables S1-S6). Based on the presence of these codes, patients were categorized into four groups: NAV only, ION only, NAV and ION, and no NAV or ION. Annual rates of NAV and ION usage in the Marketscan® population were calculated and presented as frequencies and percentages. Subsequently, analyses were undertaken to determine whether complications (including neurological injuries), 90-day readmissions, and reoperations for pedicle screw revision within 90 days of the index surgery differed among the groups. Complications within 90 days were identified with ICD-9 and CPT codes based on a validated algorithm developed by Ratliff et al. (16). This project is a secondary use of existing deidentified data. Therefore the IRB has determined that it does not meet the definition of human subject research as defined in federal regulations at 45 CFR 46.102 and is exempt from IRB review.
Statistical analysis
Univariate differences in the rates of complications were first assessed with chi-squared tests. Subsequently, the odds for neurological complications within 90 days of surgery were assessed with risk-adjusted multivariable logistic regression models, adjusting for patient demographics (age, sex, region of the country, rural/urban residence, year of surgery), comorbidities, number of surgical levels and surgical diagnosis. When necessary, post-hoc tests were conducted pairwise and corrected for multiple comparisons with the Tukey-Kramer method. Results are presented as odds ratios (OR) and 95% confidence intervals (CI). A parallel analysis was performed for the odds of having any complication within 90 days of surgery. All analyses were performed with SAS version 9.4 (Cary, NC, USA) with a two-sided level of significance of α =0.05.
Results
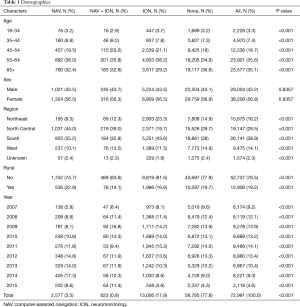
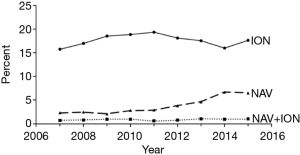
During the study period, 2007–2015, 72,991 patients underwent PLFs, of which 67,264 had continuous health plan enrollment for at least 90 days post-surgery, allowing for tracking and analysis of 90-day complications and reoperation. The average age of patients was 60.3±12.6 years, with 56.8% of patients being female. NAV only was used in 3.5% of patients, ION only in 17.9% and both NAV and ION in 0.8% of patients (Table 1). Among them, 78.5% of cases were performed in a non-rural area. During the study period, NAV only was used in 3.5% of cases, ION only was used in 17.9% of cases, and simultaneous use of NAV and ION was used in 0.8% of cases. Overall, there was a nearly three-fold increase in the use of NAV only for PLFs during the study period from 2.3% in 2007 to 6.5% in 2015 (P<0.001) (Figure 1). No significant trends were seen with ION only or both NAV and ION groups.
Full table
Clinical variables
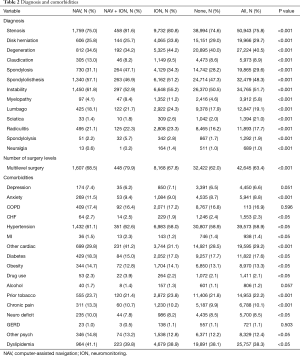
The differences in the distribution of spinal diagnosis and patient co-morbidities amongst the groups are described in Table 2. The most common spinal diagnosis was stenosis with overall prevalence of 75.8% for patients included in the study. Hypertension (58.9%), dyslipidemia (38.3%), and cardiac disease other than CHF (29.2%) were the most common co-morbidities amongst all patients.
Full table
Complications
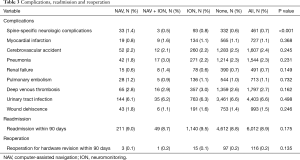
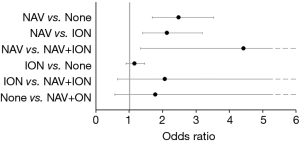
In univariate analyses, the risk of neurologic complications differed among groups (NAV only: 1.4%, ION only: 0.8%, NAV and ION: 0.5%, No NAV or ION: 0.6%, P<0.001) (Table 3). After adjusting for demographics, comorbidities, and surgical factors, the risk of neurologic complications remained different among groups, with differences found to be statistically significant. Specifically, the risk of neurological complications was higher in patients in the NAV only group compared to ION only and no ION or NAV groups [NAV vs. ION only: OR and 95% CI =2.1 (1.4, 3.2), P=0.002; NAV vs. no ION or NAV: OR and 95% CI =2.5 (1.7, 3.5), P<0.001] (Figure 2).
Full table
Despite differences in neurological complications, there were no statistically significant differences among any of the other medical complications including myocardia infarction, cerebrovascular accident, pneumonia, renal failure, pulmonary embolism, deep venous thrombosis, urinary tract infection, and wound dehiscence (P>0.10 for each) (Table 3).
Readmission and reoperations
There was no statistically significant difference in the risk of reoperation for pedicle screw revision within 90 days from surgery among the groups [NAV only 0.1%, ION only 0.1%, NAV and ION 0.2%, P=0.135] (Table 3). This result did not change in multivariable models adjusting for demographics, comorbidities, and surgical factors (P=0.315) (Figure 3). In addition, there was no difference in the rate of all-cause readmissions within 90 days among groups [NAV only 9.0%, ION only 9.5%, NAV and ION 8.7%, P=0.175] (Table 3).
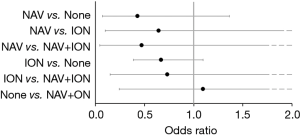
Odds ratios and 95% confidence intervals for reoperation for hardware failure within 90 days from multivariable models adjusting for patient demographics, comorbidities, and surgical factors. NAV, computer-assisted navigation; ION, neuromonitoring.
Discussion
Advancement in operative techniques for the treatment of spinal pathology aims to improve patient outcomes for treatment of complex spinal diseases. Increasingly incorporated into the development of these techniques are applied technologies aiding surgeons in performing safer and more efficient surgeries. ION and NAV are two valuable tools that have shown to decrease the risk of neurological injury in spinal deformity surgery (12,14). Since its first description in 1972, ION has been increasingly adopted by spine surgeons and considered a standard of care in deformity surgery to improve the accuracy and safety of pedicle screw implantation as well as patient safety (17). With the advancement of computing technology and imaging capabilities, NAV has also become an important adjunct for spinal surgery in the more recent years in order to decrease the frequency of pedicle screw misplacement and minimize potential associated neurological morbidity (18-20).
While the utility of ION and NAV in the surgical treatment of complex spinal deformity, trauma, and tumor resection is well established, less frequently examined is their routine use in PLF for degenerative lumbar conditions, which remains controversial. Additionally, there is a dearth of literature directly comparing outcomes (i.e., trends, complications and reoperations) of ION to NAV.
In this retrospective national database study, we found that there is a significant increase in the use of NAV for PLFs during the study period (2007 to 2015). In addition, the use of NAV only was associated with a higher risk of neurological complications when compared to ION only or no ION or NAV. However, there was no difference in reoperation rates for pedicle screw removal or revision among all groups.
Neurologic injury resulting from PLF has been evaluated in several studies. In this study, the overall risk of neurological injuries was 0.7%. This result is comparable to the findings of Ghobrial et al. who performed a literature review of iatrogenic neurologic complications in posterior decompression and fusion procedures (21). The authors reported a 1.9% rate of overall neurologic injury and a rate of 0.5% due to screw malposition. In a large retrospective database study by Kalanithi et al., the risk of neurologic injury was reported to be 0.8% amongst 66,601 posterior lumbar fusion procedures, including PLF, posterior interbody fusion, and transforaminal interbody fusion, for patients with acquired spondylolisthesis (2). It should be noted that posterior lumbar interbody fusions, which were excluded from our analysis, are associated with a higher rate of iatrogenic nerve injury up to 13.6% due to retraction of the dural sac to access intervertebral disc space (22-24). Other studies have reported an overall neurologic injury related to PLF to be as high as 7.8% and neurologic injury related to screw malposition to be as high as 2.4% (25-28). The lower rate of neurologic injury in our study is most likely attributable to our strict inclusion and exclusion criteria. For example, our study excluded all interbody fusions, posterolateral thoracic fusions, osteotomies, and corpectomies.
Although the overall risk of neurological injury observed in our study was 0.7%, the risk of neurological injury in the NAV only group was noted to be 1.4%. In addition, using multivariate logistic regression model adjusting for patient demographics, comorbidities, number of surgery levels and surgical diagnosis, the use of NAV only was associated with a higher risk of neurological complications when compared to ION only or no ION or NAV. However, there was no difference in reoperation rates for pedicle screw removal or revision among all groups which may indicate neurologic injury other than pedicle screw malposition. While multiple studies have been published on the accuracy of pedicle screw placement using NAV, there is a dearth of literature evaluating the risk of neurological injury especially in patients undergoing PLF. In a study of 100 patients undergoing PLF with and without NAV, Laine et al. reported a neurological injury rate of 0% in the NAV group and 4% in the no NAV group (19). However, the authors reported that the neurological injuries in the no NAV group were unrelated to pedicle screw placement. Chan et al. reported on screw-related complications rates in patients undergoing posterior surgery for adolescent idiopathic scoliosis (which is a patient group excluded from our study) (14). The authors reported conflicting results with regards to screw-related complications rates with some studies showing no difference between patients in the NAV group and no NAV group while other studies showed a slightly higher risk of complications in the no NAV group.
The utilization of NAV in spine surgery is of great interest to the spine community. A 2013 survey assessed the utilization of navigation among Arbeitsgemeinschaft für Osteosynthesefragen (AO) spine surgeons around the world (29). Overall, 66% of surgeons never use NAV for spinal fusions, 9% were routine users, and the remaining 25% used NAV for selected cases only. In the same study, more than 75% of all surgeons considered minimally-invasive surgery, revision cases, deformity surgery, and thoracic spine surgery (all of which are surgeries excluded from our study), as areas that would most likely benefit from NAV. If one assumes that NAV is preferentially used in select cases, this may suggest that the increase in neurological complications observed in the NAV only group in our study may be due to the fact that cases of higher complexity were disproportionately represented in the NAV group compared to the other groups. We speculate that anatomical complexity and disease severity may place some of these patients at higher risks for neurological complications from causes other than pedicle screw malposition, resulting in a patient selection bias. We also hypothesize that inexperienced surgeons may rely on navigation more frequently than their more experienced colleagues. This theory would attribute inexperience as the cause for the increased neurological complications in the NAV group. Similarly, adaptation of new technology even by experienced surgeons requires a learning curve as with a previous retrospective review showing a greater than two-fold decrease in the rate of pedicle breach using NAV over the course of 150 patients (30). Given that NAV is altogether relatively new to the field of spine surgery with incidence of use increasing more so in the last decade, this learning curve may also be responsible for the statistically significant increased incidence of neurologic injury associated with its use during the time of early adoption. For all the aforementioned reasons, it cannot be overstated that NAV use in and of itself leads to increased risk of neurologic injury.
More studies are needed to truly assess the increased incidence of neurologic injury associated with navigation as found in this study. Further evaluation of surgeons’ indications for using navigation and details of the complexity of the underlying diagnosis and procedure performed are required to determine if navigation is preferentially used for more complex cases. Additionally, in order to determine if surgeon inexperience is associated with increased NAV use or contributes to higher neurological complication rate with NAV, surgeons’ years of training and years of experience with NAV at the time of the surgery should also be evaluated. A single established surgeon’s experience with and without NAV would eliminate these possible confounders. However given the low incidence of neurologic complications, a single surgeon’s experience would likely be underpowered. Furthermore, assessment of intra-operative neuromonitoring signal changes and intra-operative revision of pedicle screws during the index procedure should also be evaluated, as breech detected intra-operatively may not be tracked as revision of pedicle screw placement, but still result in neurologic injury.
Limitations
There are some inherent limitations to this administrative database study. Significant clinical information including disease severity, aberrant anatomy, presence of a deformity, surgeon experience, and surgery complexity are not retained in the database and therefore cannot be examined. Specific characteristics of neurologic injury (i.e., transient versus permanent, radiculopathy versus cauda equina injury) are also not maintained in the database and cannot be discussed further. With a lack of coding for these specific factors, a selection bias of both complex patients and inexperienced surgeons may be at play. Finally, the interpretation of the information in the MarketScan® database relies on the accuracy of the codes, which is influenced by surgeons and quality of medical coders. Despite these recognized limitations, we believe that this study provides valuable and timely information on clinical practices in the use ION and/or NAV for PLFs in the United States, where justifying healthcare costs for improved patient outcomes has become a central issue.
Conclusions
In this retrospective national administrative database review, we found that there is a trend of increased use of NAV for PLFs during the study period. In addition, the overall risk of neurological complications following primary PLFs is low. However, the use of NAV only was associated with increased risk of neurological complications. These neurological complications are likely from reasons other than pedicle screw malposition alone since no difference in pedicle screw revision was observed among all groups.
Acknowledgments
Data for this project were accessed using the Stanford Center for Population Health Sciences Data Core. The PHS Data Core is supported by a National Institutes of Health National Center for Advancing Translational Science Clinical and Translational Science Award (UL1 TR001085) and from Internal Stanford funding.
Footnote
Conflicts of Interest: I Cheng—Nuvasive, Royalties, consulting; Globus Medical, Royalties; Spine Wave, Royalties; SpineCraft, Royalties; Cytonics, Stock; Spine Innovations, Stock; SpinalCyte, Stock. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Carreon LY, Puno RM, Dimar JR 2nd, et al. Perioperative complications of posterior lumbar decompression and arthrodesis in older adults. J Bone Joint Surg Am 2003;85:2089-92. [Crossref] [PubMed]
- Kalanithi PS, Patil CG, Boakye M. National complication rates and disposition after posterior lumbar fusion for acquired spondylolisthesis. Spine 2009;34:1963-9. [Crossref] [PubMed]
- Kimura I, Shingu H, Murata M, et al. Lumbar posterolateral fusion alone or with transpedicular instrumentation in L4--L5 degenerative spondylolisthesis. J Spinal Disord 2001;14:301-10. [Crossref] [PubMed]
- Dawson EG, Sherman JE, Kanim LE, et al. Spinal cord monitoring. Results of the Scoliosis Research Society and the European Spinal Deformity Society survey. Spine 1991;16:S361-4. [Crossref] [PubMed]
- Diab M, Smith AR, Kuklo TR. Neural complications in the surgical treatment of adolescent idiopathic scoliosis. Spine 2007;32:2759-63. [Crossref] [PubMed]
- Eggspuehler A, Sutter MA, Grob D, et al. Multimodal intraoperative monitoring during surgery of spinal deformities in 217 patients. Eur Spine J 2007;16 Suppl 2:S188-96. [Crossref] [PubMed]
- Forbes HJ, Allen PW, Waller CS, et al. Spinal cord monitoring in scoliosis surgery. Experience with 1168 cases. J Bone Joint Surg Br 1991;73:487-91. [Crossref] [PubMed]
- Kamerlink JR, Errico T, Xavier S, et al. Major intraoperative neurologic monitoring deficits in consecutive pediatric and adult spinal deformity patients at one institution. Spine 2010;35:240-5. [Crossref] [PubMed]
- Nuwer MR, Emerson RG, Galloway G, et al. Evidence-based guideline update: intraoperative spinal monitoring with somatosensory and transcranial electrical motor evoked potentials*. J Clin Neurophysiol 2012;29:101-8. [Crossref] [PubMed]
- Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 15: electrophysiological monitoring and lumbar fusion. J Neurosurg Spine 2005;2:725-32. [Crossref] [PubMed]
- Sharan A, Groff MW, Dailey AT, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 15: electrophysiological monitoring and lumbar fusion. J Neurosurg Spine 2014;21:102-5. [Crossref] [PubMed]
- Zhuang Q, Wang S, Zhang J, et al. How to make the best use of intraoperative motor evoked potential monitoring? Experience in 1162 consecutive spinal deformity surgical procedures. Spine 2014;39:E1425-32. [Crossref] [PubMed]
- Rajasekaran S, Vidyadhara S, Ramesh P, et al. Randomized clinical study to compare the accuracy of navigated and non-navigated thoracic pedicle screws in deformity correction surgeries. Spine 2007;32:E56-64. [Crossref] [PubMed]
- Chan A, Parent E, Narvacan K, et al. Intraoperative image guidance compared with free-hand methods in adolescent idiopathic scoliosis posterior spinal surgery: a systematic review on screw-related complications and breach rates. Spine J 2017;17:1215-29. [Crossref] [PubMed]
- Quraishi NA, Lewis SJ, Kelleher MO, et al. Intraoperative multimodality monitoring in adult spinal deformity: analysis of a prospective series of one hundred two cases with independent evaluation. Spine 2009;34:1504-12. [Crossref] [PubMed]
- Ratliff JK, Balise R, Veeravagu A, et al. Predicting Occurrence of Spine Surgery Complications Using "Big Data" Modeling of an Administrative Claims Database. J Bone Joint Surg Am 2016;98:824-34. [Crossref] [PubMed]
- Croft TJ, Brodkey JS, Nulsen FE. Reversible spinal cord trauma: a model for electrical monitoring of spinal cord function. J Neurosurg 1972;36:402-6. [Crossref] [PubMed]
- Kosmopoulos V, Schizas C. Pedicle screw placement accuracy: a meta-analysis. Spine 2007;32:E111-20. [Crossref] [PubMed]
- Laine T, Lund T, Ylikoski M, et al. Accuracy of pedicle screw insertion with and without computer assistance: a randomised controlled clinical study in 100 consecutive patients. Eur Spine J 2000;9:235-40. [Crossref] [PubMed]
- Tian NF, Huang QS, Zhou P, et al. Pedicle screw insertion accuracy with different assisted methods: a systematic review and meta-analysis of comparative studies. Eur Spine J 2011;20:846-59. [Crossref] [PubMed]
- Ghobrial GM, Williams KA Jr, Arnold P, et al. Iatrogenic neurologic deficit after lumbar spine surgery: A review. Clin Neurol Neurosurg 2015;139:76-80. [Crossref] [PubMed]
- Kim KT, Lee SH, Lee YH, et al. Clinical outcomes of 3 fusion methods through the posterior approach in the lumbar spine. Spine 2006;31:1351-7; discussion 1358. [Crossref] [PubMed]
- Barnes B, Rodts GE Jr, Haid RW Jr, et al. Allograft implants for posterior lumbar interbody fusion: results comparing cylindrical dowels and impacted wedges. Neurosurgery 2002;51:1191-8; discussion 1198. [Crossref] [PubMed]
- Faundez AA, Schwender JD, Safriel Y, et al. Clinical and radiological outcome of anterior-posterior fusion versus transforaminal lumbar interbody fusion for symptomatic disc degeneration: a retrospective comparative study of 133 patients. Eur Spine J 2009;18:203-11. [Crossref] [PubMed]
- Thomsen K, Christensen FB, Eiskjaer SP, et al. 1997 Volvo Award winner in clinical studies. The effect of pedicle screw instrumentation on functional outcome and fusion rates in posterolateral lumbar spinal fusion: a prospective, randomized clinical study. Spine 1997;22:2813-22. [Crossref] [PubMed]
- Cho KJ, Suk SI, Park SR, et al. Complications in posterior fusion and instrumentation for degenerative lumbar scoliosis. Spine 2007;32:2232-7. [Crossref] [PubMed]
- Mehta VA, McGirt MJ, Garces Ambrossi GL, et al. Trans-foraminal versus posterior lumbar interbody fusion: comparison of surgical morbidity. Neurol Res 2011;33:38-42. [Crossref] [PubMed]
- Patel AA, Zfass-Mendez M, Lebwohl NH, et al. Minimally Invasive Versus Open Lumbar Fusion: A Comparison of Blood Loss, Surgical Complications, and Hospital Course. Iowa Orthop J 2015;35:130-4. [PubMed]
- Härtl R, Lam KS, Wang J, et al. Worldwide survey on the use of navigation in spine surgery. World Neurosurg 2013;79:162-72. [Crossref] [PubMed]
- Wood MJ, McMillen J. The surgical learning curve and accuracy of minimally invasive lumbar pedicle screw placement using CT based computer-assisted navigation plus continuous electromyography monitoring – a retrospective review of 627 screws in 150 patients. Int J Spine Surg 2014;8:27. [Crossref] [PubMed]


