
Minimal invasive surgical algorithm for revision lumbar spinal surgery
Introduction
Elective lumbar fusion surgery in developed countries is on the rise (1), which may be a reflection of the ageing population leading to an increased prevalence of spinal pathologies, improve understanding of the indications for surgical treatment and improved perioperative care for elderly patients with multiple comorbidities. With the increase in spinal fusion rate, it is expected that the rate for revision spinal surgery will also increase over time. Revision rates following primary spinal fusion procedure ranged from 8–45%, which increase with greater follow-up (2-6). There are multiple reasons for revision surgery and diagnoses may overlap. They include recurrence of stenosis, non-union, implant failure, infection, adjacent segment disease and flat back fusion (2-6). Revision surgery can be challenging for the treating surgeon technically, but is also associated with higher procedure related complications and longer hospital stay despite similar baseline comorbidities (7). Minimally invasive surgery (MIS) techniques for managing spinal conditions have been developed to reduce complication rates, and several authors have previously published their algorithm for treating primary adult spinal deformity (ASD) using multiple MIS techniques and technologies (8-10). The use of MIS techniques for revision spinal surgery remains controversial. Our institution is a quaternary referral centre for revision and complex spinal cases in the United Kingdom and revision spinal surgeries account for half of the senior author’s (RL) practice. Since 2012, we have been using various MIS techniques for treating patients requiring revision spinal surgery. This article outlines our algorithm for selecting the appropriate MIS techniques for revision spinal surgery and present representative cases.
Methods
Preoperative assessment and planning
We routinely perform through history taking and physical examination to identify reasons for revision surgery, current symptoms, patient’s expectation and previous non-operative management. Every patient is evaluated using whole spine standing radiographs and if possible, low dose full body biplanar stereo-radiographic imaging (EOS imaging, Paris, France). MRI is obtained to assess for neural compression and CT is obtained in patients with previous fusion to assess for previous implant positioning, bone stock and presence of surgical union. It is also performed in patients with deformity to assess for auto-fusion or locked/hypertrophic facets, which may interfere with deformity correction through anterior column reconstruction using interbody cages.
Patients with risk factors for osteoporosis are also evaluated with a bone density scan. Patients who have osteoporosis (T score < minus 2.5) are deferred until bone density is optimised by a rheumatologist prior to surgery. In patients with osteopenia (T score between minus 1.5–2.5), fenestrated screws and cement augmentation may be used if we are concerned about screw purchase intra-operatively.
Algorithm design and classification
Our revision algorithm (Figure 1) is designed to guide surgeons in deciding which MIS surgical techniques to utilise in the setting of revision spinal surgery. Surgical options range from decompression employing MIS techniques to open osteotomies, but the optimal approach comes down to two deciding factors: (I) nature of previous surgery and (II) spinopelvic parameters, which are key predictors for functional outcomes in patients with ASD.
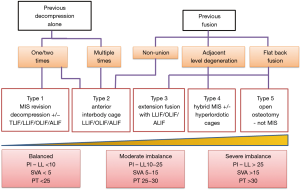
In going through our algorithm, patients are divided into two broad groups based on the nature for revision surgery. The first are patients who only had previous decompression alone (without fusion). They are then subdivided based on number for prior decompressions and spinopelvic parameters into type 1 and type 2.
Type 1 techniques are reserved for patients with normal spinopelvic parameters. They may have one to two prior decompressions. These patients typically undergo revision for recurrence of disc herniation or stenosis with symptomatic radiculopathy or neurogenic claudication. Radiographs should be carefully assessed for instability. In the absence of segmental instability, the goal for surgery is to relieve neural elements from compression and we recommend revision decompression either through standard approaches or through MIS technique using expandable retractors. If there is segmental instability on radiographs then we recommend additional stabilisation with interbody cages and screws at the listhetic level. Our preference is to perform MIS transforaminal interbody fusion through mini-Wiltse approach supplementing with percutaneous screws. Even though this group of patients may have normal spinopelvic parameters, it is still important that when short segment fusion is performed, segmental lordosis is maintained to avoid introducing iatrogenic deformity that may subsequently increase the risk of patients developing adjacent segmental degeneration. We recommend any MIS interbody fusion techniques that a surgeon is comfortable with (e.g., MIS-TLIF, LLIF, OLIF, ALIF) for this group of patients as long as segmental lordosis is maintained.
Type 2 techniques are for patients with sagittal imbalance. Patients in this group usually have mild to moderate sagittal deformity with SVA between 5–15 cm and pelvic incidence minus lumbar lordosis (PI-LL) mismatch ≤25°. These patients typically have multiple decompressions in the past, and have symptomatic axial back pain from sagittal malalignment in addition to neural compression from central canal, lateral recess and foraminal stenosis. The aim of surgery here is not only to relief neural compression but also to restore sagittal alignment. We recommend anterior column reconstruction techniques with either anterior lumbar interbody fusion (ALIF), oblique lumbar interbody fusion (OLIF) or lateral lumbar interbody fusion (LLIF) as anteriorly based techniques provide better access to insert larger lordotic cages for sagittal correction. Our preference is to perform ALIF for L5/S1 segment and OLIF for proximal lumbar segments if the patient’s anatomy allows for such technique. Anterior column reconstruction also has the benefit of indirect decompression of the foraminae and lateral recess, thus reducing the risk of dural tear in the revision setting. However, we will undertake formal revision posterior decompression if neural elements cannot be decompressed through indirect techniques using interbody cages e.g., central stenosis. We supplement our anterior construct with pedicle screws posteriorly when performing multilevel fusion or when formal decompression is performed.
The second group of patients undergoing revision are those that previously had formal surgical fusion. They are subcategorised into reasons for revision: nonunion, adjacent level degeneration and flat back fusion. Occasionally reasons for revision surgery can overlap, for example patients with previous flat back fusion may also have adjacent level degeneration.
Patients who have non-union from previous surgery can be treated using type 2 techniques. The goals here are to achieve solid union and relief of neural element compression. Anteriorly based approaches and fusion techniques are used to improve fusion rate and also allow access to remove previous interbody devices when present. Posterior screws may need to be upsized or trajectory redirected if there are signs of radiolucency. Occasionally fusion may have to be extended proximally and distally to restore sagittal alignment or to increase stability of the construct.
For patients undergoing revision surgery for adjacent level degeneration or flat back fusion or both, techniques selection ultimately depends on their spinopelvic parameters.
Type 3 techniques are chosen for patients with previous solid fusion who have moderate sagittal imbalance (SVA between 5–15 cm and PI-LL mismatch ≤25°). The deformity lies predominantly at adjacent levels from degeneration and lumbar lordosis at the fused level is relatively preserved. Surgical techniques typically involve extension fusion with ALIF, OLIF or LLIF. Our preference is to utilise standard 8° lordotic cages at L1−L3 segments and around 12−15 degrees lordotic cages at L4−S1 segments and followed by posterior screws to span anterior construct.
Type 4 techniques are reserved for patients with severe sagittal imbalance (SVA >15 cm and PI-LL mismatch >25°). Patients typically have been surgically fused with minimal lumbar lordosis and often also have adjacent levels degeneration. Instead of taking down the previously solidly fused segment, we aim to regain lumbar lordosis and sagittal correction at adjacent non-fused segments proximal and distally. For this group of patients, sequential multilevel anterior reconstruction using hyperlordotic cages through the oblique or lateral approach for proximal segments (typically 15° or 22° cages) and anterior approach for L5/S1 segment (typically >15° cages) are utilized to correct the deformity. Preoperatively the CT scan is carefully assessed to ensure facets are not “locked” or fused to allow correction using hyperlordotic cages, and if so, posterior facetectomy or removal of pre-existing implants might have to undertake prior to anterior reconstructive procedure. The procedure is planned using preoperative planning software (Surgimap, Nemaris Inc. New York, USA) to assist with the degree of cage selection preoperatively. We perform selective anterior longitudinal ligament (ALL) release at levels below L2/3 to maximise ability to restore sagittal alignment. Patients are sometimes staged between the anterior based procedure and posterior procedure. This is to allow clinical assessment for symptomatic improvement between stages; and also allow MRI and upright full spine radiographs to be re-obtained. MRI is to assess the extent of neural element decompression through indirect measures, while radiographs allow second stage to be adjusted based on the new parameters. Second stage posterior instrumented fusion is performed typically one week later. Pedicle screws are inserted through fascial stab and facet decortication performed through mini-Wiltse muscle sparing approach. If insufficient correction was obtained through anterior column reconstruction, then we add on posterior column osteotomy during our second stage. Addition correction can be achieved through a combination of extensive facetectomy, posterior column osteotomy and reduction onto contoured rod.
Type 5 techniques involve traditional open osteotomy to correct for severe sagittal deformity. The difference between patients suitable for type 4 techniques and type 5 techniques is that type 5 patients typically have previous multilevel flat back fusion and restoration of sagittal balance cannot be achieved through anterior column reconstruction at the remaining levels. Hence the only available option is to perform three column osteotomies to realign the spine through previously fused segments albeit high complication rates.
Results
Case example (Figure 2): type 1 (revision for previous decompression)
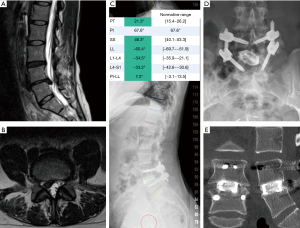
This is a 42-year-old male who had previous decompression and presented with recurrent of L4/5 disc herniation resulting in predominant right sided leg pain (Figure 2A,B). His spinal parameters were preserved. He underwent revision discectomy, MIS TLIF through right sided approach (Figure 2C,D). Postoperatively patient had complete resolution of leg pain and CT scan at 1-year demonstrating union (Figure 2E).
Case example (Figure 3): type 2 (revision for previous decompression)
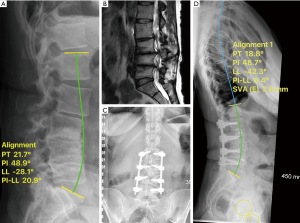
This is a 62-year-old female who previously had multiple decompressions and presented with severe back and right leg pain. Her radiographs showed loss of lumbar lordosis. Her spinopelvic parameters were: LL 28°, PI 48°, PI-LL 20°, PT 21° (Figure 3A). MRI revealed multilevel central, lateral recess and foraminal stenosis at the concavity (Figure 3B). She underwent L2/3, L3/4, L4/5 lateral lumbar interbody fusion with 8° lordotic cages followed by MIS posterior L2-L5 instrumentation. Her parameters improved to SVA 2.6 mm, LL 42°, PI-LL 6°, PT 18° (Figure 3C,D). Postoperatively patient had resolution of leg pain and improvement of her back pain.
Case example (Figure 4): type 2 (revision for non-union)
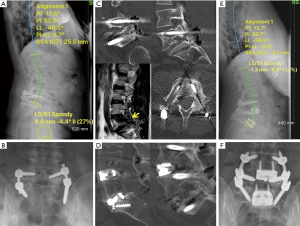
This is a 42-year-old male who previously had posterolateral instrumented fusion for L5/S1 spondylolisthesis (Figure 4A,B). He presented with persistent back and leg pain, which was no better after initial surgery. His CT scan showed screws loosening, non-union and persistent L5/S1 foraminal stenosis and adjacent L4/5 disc degeneration on MRI (Figure 4C). His spinopelvic parameters were preserved. He underwent revision L5/S1 ALIF and reduction of spondylolisthesis, indirect foraminal decompression and also ALIF of L4/5 with posterior L4−S1 instrumentation. Posterior screws were upsized to achieve satisfactory purchase (Figure 4E,F). Postoperatively patient had resolution of leg pain and improvement of his back pain and CT scan at 1-year demonstrating union (Figure 4D).
Case example (Figure 5): type 3 (adjacent segment degeneration)
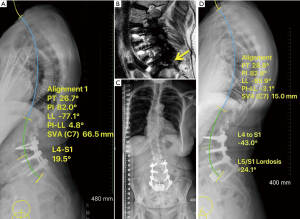
This is a 65-year-old female who previously had L3−L5 fusion (Figure 5A). She presented with severe bilateral leg pain due to adjacent segment degeneration and spondylolisthesis of L5/S1 and L5/S1 foraminal stenosis (Figure 5B). Her spinopelvic parameters were: SVA +6.6 cm, LL 77°, PI 82°, PI-LL 4°, PT 26° (Figure 5A). Even though there was no significant PI-LL mismatch, L4−S1 segment only had a lordosis of 19° (predicted 43°) due to previously flat fusion and L5/S1 degeneration. She had a compensatory hyperlordosis of L1−L3. She underwent L5/S1 ALIF with expandable hyperlordotic cage to achieve an intraoperative lordotic correction of 24°, reduction of spondylolisthesis and indirect foraminal decompression, and revision posterior instrumented fusion of L3−S1. Postoperative imaging revealed normalisation of L1−L3 segments (Figure 5C,D). Patient had resolution of leg pain and improvement of her back pain.
Case example (Figure 6): type 4 (flat back fusion, adjacent segment degeneration)
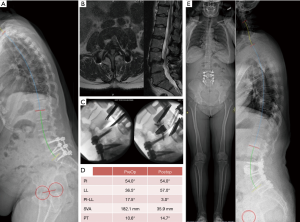
This is a 53-year-old female who previously had L4-S1 fusion. She presented with severe leg and back pain and inability to assume upright posture. Imaging revealed a flat L4−S1 fusion with adjacent segment degeneration (Figure 6A,B). Her spinopelvic parameters were: SVA +182 mm, LL 36°, PI 54°, PI-LL 18°, PT 10°. She underwent L3/4 OLIF with ALL release and reconstruction with 22° lordotic cage and plate (Figure 6C), followed by posterior extension instrumented fusion from L3 - S1. Her parameters improved to SVA 35mm, LL 57°, PI-LL 3°, PT 15° (Figure 6D,E). Postoperatively patient had resolution of leg pain and improvement of her back pain.
Case example (Figure 7): type 4 (flat back fusion, adjacent segment degeneration)
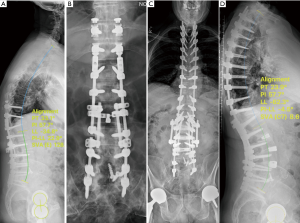
This is a 69-year-old male who previously had 7 operations with failure to correct sagittal alignment (Figure 7A,B). He presented with severe back pain and inability to maintain an upright posture. CT scan revealed solidly fused L4−S1 flat segments. His spinopelvic parameters were: SVA +12 cm, LL 34°, PI 57°, PI-LL 22°, PT 33° (Figure 7A). He underwent staged spinal reconstruction. First stage involved removal of all posterior metalwork, upsized with new screws, and anterior L1/2, L2/3, L3/4 lateral lumbar interbody fusion with lordotic cages, followed by posterior instrumented fusion T2-pelvis with bilateral facetecomies of L1/2, L2/3, L3/4 in the second stage. His parameters improved to SVA 8 mm, LL 62°, PI-LL −4°, PT 23° (Figure 7C,D). Postoperatively the patient had improvement of his back pain and posture.
Discussion
Revision spinal surgery can be technically challenging and even more so when patient has ASD as it is difficult to achieve deformity correction after previous fusion. A previous study had suggested that patients who had more previous operations (≥2) tend to present not only with worse coronal balance but also worse sagittal balance with significantly worse preoperative functional scores than those with less operations (<2) (11). Patients undergoing revision spinal surgery have overall higher risk of procedure related complications (7), however majority of patients can still anticipate improvement of their clinical symptoms at long term follow-up (11). Traditional open surgical techniques for patients with ASD have been associated with overall complication rates of 40–86% (8,12) and an 8.4% risk of major perioperative complication (13). MIS approaches have been popularised to minimise complication, however the use of MIS techniques for revision cases remain contentious. Prior reports had shown that MIS may under-correct sagittal parameters if not done well and may result in junctional failures, pseudarthrosis and predispose the patient to even more surgeries (10,14). Our algorithm for revision spinal surgery combines multiple MIS techniques already familiar to most spinal surgeons and aims to assist surgeons in selecting the most appropriate combination of MIS techniques to achieve the goals for revision surgery. We pay particular attention in restoring sagittal alignment in revision surgery using Surgimap software as part of our preoperative planning. The software simulates sagittal correction manoeuvers on the preoperative X-ray and determines the effect of anterior column correction on spinopelvic and global alignment. We also take into account patients’ age in our operative realignment targets, restoring alignment to parameters that is appropriate for patients’ age group (15). Elderly patients may naturally have an increased SVA and pelvic retroversion and hence need less rigorous correction compared to younger patients (15). In addition, for patients without prior fusion (type 1 and type 2 cases), we pay particular attention to restoring normal spinal harmony. In patients requiring short segment fusion, restoring segmental lordosis is important in preventing adjacent segment degeneration (16). It is generally accepted that the L4−S1 segment should account for approximately two-thirds of total lumbar lordosis (17,18). A recent publication by Anwar et al. (19) has improved our understanding of the distribution of segmental lumbar lordosis based on Roussouly spine classification (18,20). According to the authors, for Roussouly type 1 and type 2 spine, the apex of lordosis is low in the lumbar spine and most of the lumbar lordosis arises between L4−S1 segment (19), and therefore the principle of restoring 66% of total lordosis in that segment holds true. The L1−L2 segment may even be kyphotic in the Roussouly type 1 spine. However as pelvic incidence increases, the apex of lordosis moves cranially and proportion of lordosis provided by cephalad lumbar segments also increases and the contribution of the L4-S1 segment to the total lordosis decreases (19). Hence patients with high pelvic incidence may not need two-thirds of lumbar lordosis between L4-S1, and restoring lordosis in the proximal segments between L1-L4 is equally important in this group of patients. In patients who had previous fusion (type 3 and type 4 cases), instead of taking down prior fusion, we aim to regain lumbar lordosis and sagittal correction at adjacent non-fused segments proximal and distally.
Determining the extent of fusion remains controversial. Distally L5/S1 is included as foundation of fusion when long fusion is planned for moderate to severe deformity or when there is associated stenosis, degeneration or instability at that level. Most elderly patients often already have degenerative changes at L5/S1 segment, and studies have shown that advanced disc degeneration with instability and loss of sagittal alignment occurs in 50–58% of patients whose fusion end at L5 (21,22). In addition, anterior column reconstruction at the L5/S1 segment allows most of patient’s lordosis to be regained to restore sagittal alignment in revision surgery especially for previous flat back fusion. When long spinal construct is planned, we also prefer to instrument the pelvis to prevent distal failures. In long constructs, most of the mechanical stress is concentrated at fusion ends and evidence suggests that there is a high rate of distal junctional failures in patients with long fusion constructs (>5 levels) if distal fusion was to stop at L5 and S1 than those who had spinopelvic fixation (22). Our pelvic fixation of preference is S2-alar-iliac (S2AI) screws rather than pelvic bolts. Similar to other authors, we find that S2AI screws are often more in line with S1 pedicle screws avoiding the need for lateral connectors and as the screw heads lie deeper with better soft tissue coverage than iliac bolts, there is less need for revision due to screw prominence (23). In a recent publication by Ishida et al., the authors have confirmed our experience that S2AI technique has a lower rate of overall reoperation rates than iliac bolts (23).
Regarding selection of proximal instrumented vertebra, we span our construct to include all anterior reconstructed levels. If coronal Cobb is >20°, we will also include all vertebrae contained within the Cobb angle. Some surgeons will routinely instrument proximally to T10 when correcting moderate to severe sagittal deformity to reduce proximal junctional kyphosis (PJK). The rationale behind this is T10 segment is supported by true ribs and biomechanically stiffer than the more flexible thoracolumbar junction of T11-L2. However Kim et al. have demonstrated that proximal fusion ending at L1-L2 did not have significant radiographic, clinical outcomes or revision prevalence than those ending at T9-T10 or T11−T12 at long term follow-up provided satisfactory sagittal correction has been achieved intraoperatively (24). This is in line with our experience and we will routinely stop proximally at L1 provided satisfactory sagittal correction has been achieved through anterior column reconstruction. However, for patients where we cannot achieve sagittal correction anteriorly and need to rely on posterior instrumentation with or without posterior column osteotomies to achieve additional correction, we may extend our posterior construct into the upper thoracic region (often T2 or T4). In general, patients with PI-LL mismatch with loss of lumbar lordosis have compensatory thoracic hypokyphosis. However, some patients may present with thoracic decompensation with kyphosis and severe positive sagittal imbalance. We find that this group of patients often have poor muscular tone hence the decompensation, and this is another subset of patients that we consider extension fusion to the upper thoracic region. Our practice is confirmed by a recent article by Hyun et al. where the authors observed that patients with lower thoracolumbar muscularity have higher thoracolumbar kyphosis and at higher risk of PJK (25).
In conclusion, careful patient and technique selection is important in achieving satisfactory outcome for patient requiring revision lumbar spinal surgery. Our revision algorithm provides surgeons with a systematic approach in selecting the appropriate combination of MIS techniques based on pathology and sagittal alignment. Future studies will be needed to confirm the validity of our algorithm in relation to radiological and functional outcomes.
Acknowledgments
None.
Footnote
Conflicts of Interest: R Lee is a consultant for Stryker. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethics approval was not required for this study. Consent was obtained from all patients for their radiological and clinical data to be collected for research purposes.
References
- Martin BI, Mirza SK, Spina N, et al. Trends in Lumbar Fusion Procedure Rates and Associated Hospital Costs for Degenerative Spinal Diseases in the United States, 2004-2015. Spine (Phila Pa 1976) 2019;44:369-76. [Crossref] [PubMed]
- Lehmann TR, Spratt KF, Tozzi JE, et al. Long-term follow-up of lower lumbar fusion patients. Spine (Phila Pa 1976) 1987;12:97-104. [Crossref] [PubMed]
- Mok JM, Cloyd JM, Bradford DS, et al. Reoperation after primary fusion for adult spinal deformity: rate, reason, and timing. Spine (Phila Pa 1976) 2009;34:832-9. [Crossref] [PubMed]
- Pichelmann MA, Lenke LG, Bridwell KH, et al. Revision rates following primary adult spinal deformity surgery: six hundred forty-three consecutive patients followed-up to twenty-two years postoperative. Spine (Phila Pa 1976) 2010;35:219-26. [Crossref] [PubMed]
- Puvanesarajah V, Shen FH, Cancienne JM, et al. Risk factors for revision surgery following primary adult spinal deformity surgery in patients 65 years and older. J Neurosurg Spine 2016;25:486-93. [Crossref] [PubMed]
- Zhu F, Bao H, Liu Z, et al. Unanticipated revision surgery in adult spinal deformity: an experience with 815 cases at one institution. Spine (Phila Pa 1976) 2014;39:B36-44. [Crossref] [PubMed]
- Diebo BG, Passias PG, Marascalchi BJ, et al. Primary Versus Revision Surgery in the Setting of Adult Spinal Deformity: A Nationwide Study on 10,912 Patients. Spine (Phila Pa 1976) 2015;40:1674-80. [Crossref] [PubMed]
- Anand N, Kong C, Fessler RG. A Staged Protocol for Circumferential Minimally Invasive Surgical Correction of Adult Spinal Deformity. Neurosurgery 2017;81:733-9. [Crossref] [PubMed]
- Choy W, Miller CA, Chan AK, et al. Evolution of the Minimally Invasive Spinal Deformity Surgery Algorithm: An Evidence-Based Approach to Surgical Strategies for Deformity Correction. Neurosurg Clin N Am 2018;29:399-406. [Crossref] [PubMed]
- Mummaneni PV, Shaffrey CI, Lenke LG, et al. The minimally invasive spinal deformity surgery algorithm: a reproducible rational framework for decision making in minimally invasive spinal deformity surgery. Neurosurg Focus 2014;36:E6. [Crossref] [PubMed]
- Hu X, Lieberman IH. Revision adult spinal deformity surgery: Does the number of previous operations have a negative impact on outcome? Eur Spine J 2019;28:155-60. [Crossref] [PubMed]
- Schwab FJ, Hawkinson N, Lafage V, et al. Risk factors for major peri-operative complications in adult spinal deformity surgery: a multi-center review of 953 consecutive patients. Eur Spine J 2012;21:2603-10. [Crossref] [PubMed]
- Yeramaneni S, Robinson C, Hostin R. Impact of spine surgery complications on costs associated with management of adult spinal deformity. Curr Rev Musculoskelet Med 2016;9:327-32. [Crossref] [PubMed]
- Wang MY, Mummaneni PV. Minimally invasive surgery for thoracolumbar spinal deformity: initial clinical experience with clinical and radiographic outcomes. Neurosurg Focus 2010;28:E9. [Crossref] [PubMed]
- Lafage R, Schwab F, Challier V, et al. Defining Spino-Pelvic Alignment Thresholds: Should Operative Goals in Adult Spinal Deformity Surgery Account for Age? Spine (Phila Pa 1976) 2016;41:62-8. [Crossref] [PubMed]
- Rothenfluh DA, Mueller DA, Rothenfluh E, et al. Pelvic incidence-lumbar lordosis mismatch predisposes to adjacent segment disease after lumbar spinal fusion. Eur Spine J 2015;24:1251-8. [Crossref] [PubMed]
- Barrey C, Roussouly P, Le Huec JC, et al. Compensatory mechanisms contributing to keep the sagittal balance of the spine. Eur Spine J 2013;22 Suppl 6:S834-41. [Crossref] [PubMed]
- Roussouly P, Gollogly S, Berthonnaud E, et al. Classification of the normal variation in the sagittal alignment of the human lumbar spine and pelvis in the standing position. Spine (Phila Pa 1976) 2005;30:346-53. [Crossref] [PubMed]
- Anwar HA, Butler JS, Yarashi T, et al. Segmental Pelvic Correlation (SPeC): a novel approach to understanding sagittal plane spinal alignment. Spine J 2015;15:2518-23. [Crossref] [PubMed]
- Roussouly P, Pinheiro-Franco JL. Sagittal parameters of the spine: biomechanical approach. Eur Spine J 2011;20 Suppl 5:578-85. [Crossref] [PubMed]
- Cho KJ, Suk SI, Park SR, et al. Arthrodesis to L5 versus S1 in long instrumentation and fusion for degenerative lumbar scoliosis. Eur Spine J 2009;18:531-7. [Crossref] [PubMed]
- Yasuda T, Hasegawa T, Yamato Y, et al. Lumbosacral Junctional Failures After Long Spinal Fusion for Adult Spinal Deformity-Which Vertebra Is the Preferred Distal Instrumented Vertebra? Spine Deform 2016;4:378-84. [Crossref] [PubMed]
- Ishida W, Elder BD, Holmes C, et al. Comparison Between S2-Alar-Iliac Screw Fixation and Iliac Screw Fixation in Adult Deformity Surgery: Reoperation Rates and Spinopelvic Parameters. Global Spine J 2017;7:672-80. [Crossref] [PubMed]
- Kim YJ, Bridwell KH, Lenke LG, et al. Is the T9, T11, or L1 the more reliable proximal level after adult lumbar or lumbosacral instrumented fusion to L5 or S1? Spine (Phila Pa 1976) 2007;32:2653-61. [Crossref] [PubMed]
- Hyun SJ, Kim YJ, Rhim SC. Patients with proximal junctional kyphosis after stopping at thoracolumbar junction have lower muscularity, fatty degeneration at the thoracolumbar area. Spine J 2016;16:1095-101. [Crossref] [PubMed]


