Pediatric tethered cord release: an epidemiological and postoperative complication analysis
Introduction
Tethered cord syndrome (TCS) is a condition characterized by neurological, gastrointestinal, musculoskeletal, and urinary dysfunction attributable to spinal cord traction, with an incidence estimated at 0.25 per 1,000 births (1-5). TCS may present as an isolated finding, but is frequently a component of occult spinal dysraphism (OSD) such as fatty filum terminale (FT), split cord malformation, or dermal sinus tracts (6-8). Patients commonly present as infants or young children with cutaneous stigmata, progressive lower extremity orthopedic deformities, bowel or bladder dysfunction, progressive sensorimotor deficits, progressive scoliosis, or often, as an incidental finding (9). Imaging, particularly MRI, has become standard for visualizing the conus medullaris and assessing filum thickness during workup (4,10). Surgical treatment for tethered cord traditionally consists of surgical detethering via one level lumbar laminectomy (11). Typically the filum is identified, coagulated, and cut, with satisfactory outcomes and overall low rate of complication pending the underlying pathology. Although rare, complications may include infection, CSF leak, and nerve root or spinal cord injuries (12). OSDs reportedly demonstrate female predominance (8,13), yet there is a lack of literature describing whether differences in surgical outcomes by sex exist.
The American College of Surgeons-National Surgical Quality Improvement Program pediatric database (ACS-NSQIP-P) is a large, international, multi-institutional quality improvement database that collects several outcome variables in a standardized database. The pediatric database is a collaboration between American College of Surgeons (ACS) and American Pediatric Surgical Association (APSA). In this study, the ACS-NSQIP-P is utilized to describe epidemiology and post-operative complication analysis stratified by sex for pediatric patients undergoing release of tethered cord for primary tethered cord syndrome
Methods
Patient selection
A retrospective review of pediatric patients from ACS-NSQIP-P was performed, analyzing data collected from 2012 to 2016. The study cohort was selected by primary current procedural terminology (CPT) code 63200 (Laminectomy, with release of tethered spinal cord, lumbar), then stratified by ICD-9 code 742.59 and corresponding ICD-10 code Q06.8, which include congenital and exclude acquired tethered cord syndrome. Of note, this ICD code does not specify specific etiology (fatty filum, split cord malformation or dermal sinus tract), however ICD codes specific for myelomeningocele diagnoses were excluded. To minimize confounders, patients were excluded if they were missing information for sex, height, weight, American Society of Anesthesiology (ASA) classification, if there was a previous operation within 30 days, open wound infection, or preoperative sepsis. Patient selection with inclusion and exclusion criteria are detailed in Figure S1. Since ACS-NSQIP-P is de-identified and poses no risk to the participants, a waiver for consent was granted by the university institutional review board.
Variables analyzed
Demographic data including sex, race, age, body mass index (BMI), were analyzed, along with comorbidity data including ASA classification, organ system comorbidities, nutritional support, hematologic comorbidities, steroid use within 30 days, and active/past malignancy. Comorbidities were analyzed if identified in 5 or more patients. Age was converted to years for univariate and multivariate analysis of complications. BMI was calculated using the National Institute of Health conversion formula using height and weight data. To evaluate patient fitness prior to the operation, ASA classification was condensed into groups 1–2 and 3–4. For all Intraoperative data included the length of operation and use of operative microscope. Post-operative outcomes included unplanned readmissions, reoperation, death within 30 days, days from operation to discharge, and post-operative complications. All demographic, comorbidity, and operative data were stratified by sex.
Statistical analysis
Categorical variables were compared using Chi-square and Fisher’s Exact tests, while continuous variables were studied using t-test. Univariate and multivariate analyses were performed to evaluate risk factors for post-op complications. Multivariate regression analyzed risk factors individually while controlling for all significant risk factors found in univariate analysis and age to remove confounders. Statistical significance was set at P<0.05 and percentile values were calculated from the proportions of patients where information was available. Statistical analysis was performed using SAS (SAS Institute Inc., Cary, NC).
Results
Population demographics and comorbidities
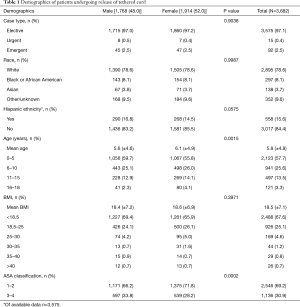
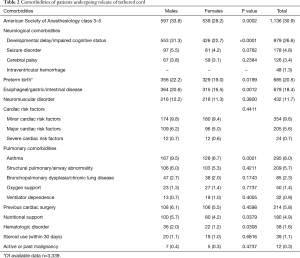
A total of 3,682 pediatric patients were studied. Demographic information is detailed in Table 1. There was a balanced distribution of males (48.0%) and females (52.0%), with most cases elective (97.1%). The mean age was 5.8 years, with a majority (57.7%) younger than 5. A majority (69.2%) of patients were relatively healthy before the surgery, having ASA classification between 1–2. Table 2 describes preoperative comorbidities. The most common comorbidity was developmental delay/impaired cognitive status (26.6%), followed by preterm birth (20.5%), gastrointestinal disease (18.4%), and neuromuscular disorder (11.7%).
Full table
Full table
Sex differences in demographics and comorbidities
Several sex associated differences were observed in demographic and comorbidity data. Males were significantly younger versus females (5.6 vs. 6.1 years, P=0.0015) and had a higher rate of illness or disability, with a greater proportion of patients with ASA classification of 3–4 (33.8% vs. 28.2%, P=0.0002). Males had significantly higher rates of developmental delay/impaired cognitive status (31.3% vs. 22.7%, P<0.0001), preterm birth (22.2% vs. 19.0%, P=0.0199), gastrointestinal disease (20.6% vs. 16.5%, P=0.0012), asthma (9.5% vs. 6.7%, P=0.0021), required preoperative nutritional support (5.7% vs. 4.2%, P=0.0379), and had hematologic disorders (2.0% vs. 1.2%, P=0.0308) compared to females. Detailed comparisons are shown in Tables 1,2.
Postoperative outcomes
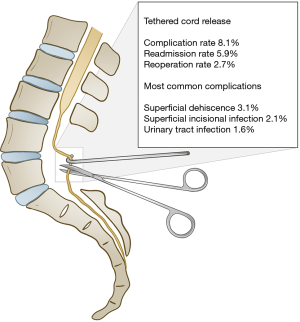
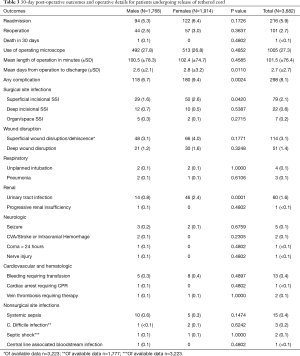
Thirty-day postoperative events are described in Table 3. The mean length of operation was 101.5 minutes, with 1,005 (27.3%) cases utilizing an operative microscope. Over the 30-day postoperative period, the readmission rate was 5.9% and reoperation rate 2.7%. The average hospital length of stay was 2.7 days. Two hundred and ninety-eight (8.1%) patients had at least one complication in the 30-day postoperative period. The most common postoperative complication was superficial wound complication (3.1%), followed by superficial incisional surgical site infection (2.1%), urinary tract infection (1.6%), and deep wound disruption (1.4%). 30-day postoperative outcomes are summarized in Figure 1.
Full table
Sex differences in postoperative outcomes
Outcomes stratified by sex identified females had a significantly longer length of hospital stay versus males (2.8 vs. 2.6 days, P=0.0110), and an increased 30-day complication rate (9.4% vs. 6.7%, P=0.0024). Of the complications noted, females had a significantly higher rate of superficial surgical site infections (2.6% vs. 1.6%, P=0.0420) and urinary tract infections (2.4% vs. 0.8%, P=0.0001). Despite having a higher rate of preoperative comorbidities, males did not show increased incidence for any postoperative complication.
Readmission and reoperation
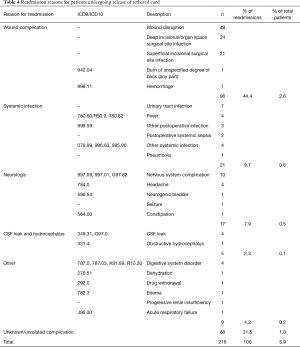
Risk factors for readmission are detailed in Table 4. The most common type of readmission was wound complication, (2.6% of all patients). Other causes included systemic infection (0.6% of all patients) and neurologic causes (0.5% of patients). Non-neurologic causes were unusual and ranged from digestive system disorders to respiratory failure. Few patients (0.1%) were readmitted for CSF leak or hydrocephalus.
Full table
Reasons for reoperation are listed in Table 5. 32.7% of all reoperations and 0.9% of all patients underwent CSF leak repair or CSF diversion postoperatively. Reoperations for infection were required in 0.6% of patients, while wound repair or debridement was required in 0.5%. Other reoperation procedures included cystourethroscopy, establishment of central venous access, removal of sutures, and removal of fecal impaction. 0.6% of patients required a reoperation that was either unrelated to tethered cord or was unknown.

Full table
Risk factors for postoperative complications
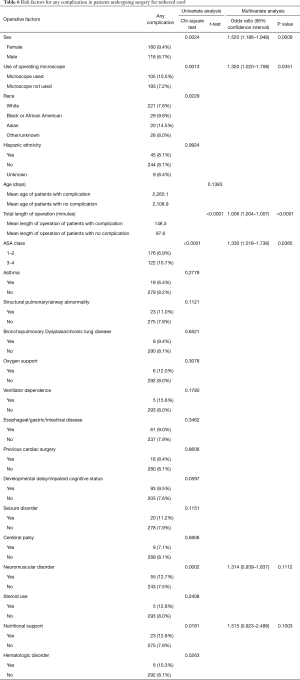
Univariate and multivariate analyses were performed to analyze demographic and comorbidity risk factors for postoperative complications. A detailed analysis of all risk factors is presented in Table 6. Univariate analysis identified risk factors associated with post-operative complications as female sex (P=0.0024), microscope use (P=0.0013), Asian race (P=0.0229), operative time (P<0.0001), ASA classification of 3–4 (P<0.0001), neuromuscular disorders (P=0.0002), and nutritional support needs (P=0.0181). A multivariate logistic regression was performed on all the significant postoperative complication risk factors in univariate analysis. The multivariate logistic regression revealed significant risk factors for postoperative complications as female sex (P=0.0009), use of an operative microscope (P=0.0351), operative time (P<0.0001), and ASA classification of 3−4 (P=0.0365).
Full table
Discussion
TCS is a common diagnosis that has become more frequent with the advancement and availability of imaging. Deciding when to offer surgical untethering is a common dilemma encountered by pediatric neurosurgeons (8,10,12). While TCR is certainly recommended once neurologic or urologic symptoms present (4,10), surgery in the asymptomatic patient with incidental finding is debated (8,10,14,15). A common argument for prophylactic procedures is that once neurologic or urologic symptoms arise, there is a realistic risk of permanent deficit despite surgical intervention (15-19). Literature reports of urologic and neurologic outcomes post detethering vary widely by OSD type and preoperative status, but the overall agreement is earlier intervention improves outcomes (4,15,19-25).
As optimal timing of operative intervention is not always clear for some patients, characterization of complications, reoperations, and readmissions for TCR is important to shine light on the risk versus benefit on a case-by-case basis. The pediatric ACS-NSQIP is a large, international quality-improvement database that tracks numerous outcome variables for surgeries in a standardized format. In this study, 30-day outcomes for 3,682 pediatric patients identified an 8.1% overall complication rate, 5.9% readmission rate, and 2.7% reoperation rate for children undergoing TCR, underscoring the relative safety of this procedure. The most frequent etiology of complication, readmission, and reoperation was wound complication. Conclusively, relative to the risk of neurological deficit, pain, progressive scoliosis, and bowel and bladder dysfunction associated with TCS, surgical risk profile associated with surgical detethering is low.
Operative details and outcomes
Tethered cord release is widely regarded as a safe procedure, reflected by the relatively short postoperative length of stay and low complication rate. The length of stay in the NSQIP population (2.7 days) is consistent with other reports, and some authors have suggested patients may be discharged 1 day postoperatively without increased risk of complication for uncomplicated TCR (26). Variability in length of stay may reflect the complexity of tethered cord etiology or whether the procedure is revision for retethered cord; unfortunately, this data analysis does not allow for distinguishing between these etiologies based on ICD codes to any meaningful degree.
The overall complication rate of 8.1% in the NSQIP cohort is also consistent with the literature, which has reported complications ranging from 1.0–11.0%, with the majority due to wound dehiscence, surgical site infections and CSF leak (12,24,27-31). The wide range in complications is likely a direct correlate of the wide range of disorders that make up the umbrella of TCS, with inherent complexity of disease processes correlating with increased complication. This cohort corroborates prior findings within the literature that wound healing is the most common complication encountered from this procedure. It is worthy to note the dreaded complication of CSF leak in this large cohort was indeed minimal and given this complication is often seen in the early postoperative period is it fair to assume that 30-day postop evaluation would accurately capture this subset. These and associated complications, including seroma and pseudomeningocele can in part be due to poor wound healing from nutritional deficiencies, other comorbidities, or less commonly poor wound closure. This can be particularly troublesome in certain types of OSD. It is important for neurosurgeons to extensively optimize patients undergoing such procedures in a collaborative effort with patient’s primary care physicians, focusing on nutritional status, comorbidities, minimizing medications that may interfere with wound healing such as steroids (i.e., in case of patients with severe asthma etc.). In cases where closure is expected to be challenging, some authors advocate for collaboration with plastic surgery for a composite closure with fascial and musculofascial flap layers, however, this is reserved for extenuating circumstances where significant tissue compromise has occurred secondary to infection, compromised blood flow, non-healing wounds, or premature infants undergoing repeat procedures for wound breakdown (28).
Sex differences in comorbidities and outcomes
Historically, most OSDs have demonstrated a female predominance (8,13), although the reasons are unclear. Of interest, the NSQIP cohort did not reflect this, and had a relatively close ratio (48.0% male, 52.0% female). Many reported comorbidities were significantly more likely in males (developmental delay, preterm birth, GI disease, asthma, nutritional support, hematological disorder), and males also exhibited a greater ASA class versus females, both previously unidentified in the literature. The significantly younger age of males compared to females (5.6 vs. 6.1 years) may be a contributor, as earlier symptom onset typically correlates with more severe OSD (8,10,13,14), but does not solely account for this finding, because statistical significance remained after controlling for age. Interestingly, the statistically significant comorbidities within the male cohort did not translate into a higher postoperative risk; women displayed a higher postoperative risk profile for postop infection such as UTI, wound healing, and length of stay. The significant increase in postoperative UTI for females can be expectedly attributed to anatomical variation (shorter urethra), which pose a higher risk of UTI at baseline in females than males at all age-groups. These findings raise an important point to consider avoiding routine use of Foley catheters in the female population.
Reoperations and readmissions
CSF leak after TCR is a commonly discussed complication, sometimes requiring readmission or reoperation, and has been reported in the literature at rates of 1.9–8% (26,28,29). In our cohort, CSF leak was less frequently encountered than reported literature and it was the most common reason for reoperation, but not for readmission; indicating typically it was diagnosed and treated prior to patient discharge. There are no guidelines for optimal duration of postoperative recumbency to minimize risk of CSF leak complications, however, previous literature has suggested that remaining flat greater than 24 hours provides no major benefit (26). Conversely, wound complication was disproportionately found as the cause for readmission versus reoperation, suggesting infections presented in a delayed fashion, and did not always require surgical correction. It is important to note that superficial wound healing complication is not synonymous with wound infection, as there are many etiologies to non-healing wound which can be resolved with conservative management. Similarly, superficial wound infection can be managed conservatively via a course of antibiotics and aggressive wound care. This may explain the disproportionate number of patients admitted for concerns of wound healing, without reoperation. Hemorrhagic and neurologic complication rates for TCR were low in this cohort, and are consistent with rates reported in the literature (28). Certain neurologic complications may further reflect the initial etiology and symptoms of tethered cord and not the procedure itself, with older symptomatic patients having a greater risk of neurologic decline (8).
Complication risk factors
The relationship between ASA class, neuromuscular disorders, need for nutritional support and complication risk was expected, and consistent with previous literature showing an association between comorbid conditions and the development of complications (12). Use of operating microscope and increased operating time were significantly correlated with risk of complication and raises the question whether these factors are associated with more challenging cases prone to complication (split cord malformation or dermal sinus tract versus fatty filum), or are risk factors for complications themselves. Though this assumption comes from experience, any further data to support correlation between these two seemingly related factors is limited. Of note, use of operating microscope itself was not associated with a longer surgical duration, and remained significant when controlling for other significant risk factors. This may reflect the surgeon’s preference in utilization of microscope, or an indication of procedural complexity independently of increased surgical duration. As previously discussed, the procedural complexity varies based on etiology of OSD, which cannot be separated within this cohort. Other studies have identified an association between age and short-term complications, specifically among older children (12,27), but this was not the case in the NSQIP cohort.
Tethered cord and the pediatric neurosurgeon
Tethered cord release is largely a safe procedure, with low readmission, reoperation, and complication rates. When complications occur, they tend to be mild and transient, such as wound complication or superficial infection, not requiring surgical reoperation. To decrease the risk of these complications, the surgeon should ensure adequate antibiotic prophylaxis and in cases of complex wound closure consider a longer course of postoperative antibiotics. In select cases with notable wound complexity, consulting with a plastic surgeon to discuss closure options and possibility of utilizing a flap for closure may be utilized; this is in essence not an extrapolation of the data, rather a tool in the armamentarium of the neurosurgeon based on their clinical judgement. With complications uncommon and mild, and high risk of progressive and potentially permanent deficits if procedures are delayed until a patient is symptomatic (15-19), we recommend the surgeon avoid excessive caution when considering surgical intervention. As the most encountered cause for reoperation is CSF leak (0.9% of cases in the cohort), water-tight dural closure is critical to avoid this potential complication. To ensure adequate dural closure, the surgeon may perform a Valsalva maneuver after dural suturing with or without graft and use dural sealants as clinically deemed fit. Inevitably, any discussion of long term follow up within this cohort is unavailable, however, the literature reflects postoperative retethering is a common occurrence, reported at rates of 8–30%; as such, long term follow up is recommended (4,10,24,32). Findings in our cohort suggest females require particular attention for postoperative UTI and surgical site infections. To help avoid these complications, Foley catheters should be avoided in shorter duration cases or removed as soon as possible, the patient positioned prone for at least 24 hours postoperatively, and the wound kept clean and free of pressure until it heals. Comorbidities are predictive of higher complication rates, as expected with any surgery, but overall the complication rate remains low when present, underscoring the relative safety of tethered cord release.
Limitations
Large database studies carry several inherent limitations, including missing data and possible coding errors of procedures and diagnoses. While ACS-NSQIP-P provides a large sample size and relevant data, it does not consider variables of interest specific to TCS patients, such as sensory and motor, urinary, or bowel dysfunction after surgery. Additionally, the ICD codes used do not specify etiology of TCS, limiting analysis in context of OSD severity. Moreover, the patient population includes both simple filum transection as well as more complex forms of OSD. This brings in question whether the use of microscope is more prevalent in simple filum resection or complex forms of OSD, as microscope use was associated with increased postoperative infections. To avoid potential selection bias, NSQIP-P uses a systematic sampling system to select completed cases from the hospital’s operative log. This ensures cases have an equal chance of being selected from each day of the week. Therefore, while the NSQIP-P database provides a large sample of pediatric tethered cord cases, it is noteworthy that it does not include all tethered cord cases from the participating institutions. Additionally, this analysis is limited to 30-day outcomes, which limits discussion of complications such as retethering.
Conclusions
Tethered cord release is a common procedure in pediatric neurosurgery with a relatively safe risk profile. This study confirms the benign nature of this surgery, with a 30-day complication rate of 8.1%, readmission rate of 5.9%, and reoperation rate of 2.7%. Paradoxically, females were found to have a higher complication rate despite males having more comorbidities during presentation. Operative time, use of the surgical microscope, and ASA class were also associated with complications. Wound complication was the most common cause for readmission, and CSF leak the most common for reoperation, although certain intraoperative and postoperative techniques may help decrease these risks. This paper provides a large sample size of multi institutional pediatric patients undergoing TCR and may serve as a contemporary “snapshot” for future studies.
Acknowledgments
The authors thank and acknowledge Zaid Zayyad from University of Illinois at Chicago College of Medicine Class of 2019 for his help with creating the figure used in this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Since ACS-NSQIP-P is de-identified and poses no risk to the participants, a waiver for consent was granted by the university institutional review board.
References
- Bademci G, Saygun M, Batay F, et al. Prevalence of primary tethered cord syndrome associated with occult spinal dysraphism in primary school children in Turkey. Pediatr Neurosurg 2006;42:4-13. [Crossref] [PubMed]
- McGirt MJ, Mehta V, Garces-Ambrossi G, et al. Pediatric tethered cord syndrome: response of scoliosis to untethering procedures. Clinical article. J Neurosurg Pediatr 2009;4:270-4. [Crossref] [PubMed]
- Saker E, Henry BM, Tomaszewski KA, et al. The filum terminale internum and externum: A comprehensive review. J Clin Neurosci 2017;40:6-13. [Crossref] [PubMed]
- Geyik M, Alptekin M, Erkutlu I, et al. Tethered cord syndrome in children: a single-center experience with 162 patients. Childs Nerv Syst 2015;31:1559-63. [Crossref] [PubMed]
- O'Neill BR, Gallegos D, Herron A, et al. Use of magnetic resonance imaging to detect occult spinal dysraphism in infants. J Neurosurg Pediatr 2017;19:217-26. [Crossref] [PubMed]
- Shukla M, Sardhara J, Sahu RN, et al. Adult Versus Pediatric Tethered Cord Syndrome: Clinicoradiological Differences and its Management. Asian J Neurosurg 2018;13:264-70. [Crossref] [PubMed]
- Hoffman HJ, Hendrick EB, Humphreys RP. The tethered spinal cord: its protean manifestations, diagnosis and surgical correction. Childs Brain 1976;2:145-55. [PubMed]
- Tuite GF, Thompson DNP, Austin PF, et al. Evaluation and management of tethered cord syndrome in occult spinal dysraphism: Recommendations from the international children's continence society. Neurourol Urodyn 2018;37:890-903. [Crossref] [PubMed]
- Dias M, Partington M. SURGERY SON. Congenital Brain and Spinal Cord Malformations and Their Associated Cutaneous Markers. Pediatrics 2015;136:e1105-19. [Crossref] [PubMed]
- Agarwalla PK, Dunn IF, Scott RM, et al. Tethered cord syndrome. Neurosurg Clin N Am 2007;18:531-47. [Crossref] [PubMed]
- Solmaz I, Izci Y, Albayrak B, et al. Tethered cord syndrome in childhood: special emphasis on the surgical technique and review of the literature with our experience. Turk Neurosurg 2011;21:516-21. [PubMed]
- Shweikeh F, Al-Khouja L, Nuño M, et al. Disparities in clinical and economic outcomes in children and adolescents following surgery for tethered cord syndrome in the United States. J Neurosurg Pediatr 2015;15:427-33. [Crossref] [PubMed]
- Sanchez T, John RM. Early identification of tethered cord syndrome: a clinical challenge. J Pediatr Health Care 2014;28:e23-33. [Crossref] [PubMed]
- Bui CJ, Tubbs RS, Oakes WJ. Tethered cord syndrome in children: a review. Neurosurg Focus 2007;23:E2. [Crossref] [PubMed]
- Satar N, Bauer SB, Shefner J, et al. The effects of delayed diagnosis and treatment in patients with an occult spinal dysraphism. J Urol 1995;154:754-8. [Crossref] [PubMed]
- Atala A, Bauer SB, Dyro FM, et al. Bladder functional changes resulting from lipomyelomeningocele repair. J Urol 1992;148:592-4. [Crossref] [PubMed]
- Proctor MR, Bauer SB, Scott RM. The effect of surgery for split spinal cord malformation on neurologic and urologic function. Pediatr Neurosurg 2000;32:13-9. [Crossref] [PubMed]
- Selçuki M, Umur AS, Duransoy YK, et al. Inappropriate surgical interventions for midline fusion defects cause secondary tethered cord symptoms: implications for natural history report of four cases. Childs Nerv Syst 2012;28:1755-60. [Crossref] [PubMed]
- Frainey BT, Yerkes EB, Menon VS, et al. Predictors of urinary continence following tethered cord release in children with occult spinal dysraphism. J Pediatr Urol 2014;10:627-33. [Crossref] [PubMed]
- Hsieh MH, Perry V, Gupta N, et al. The effects of detethering on the urodynamics profile in children with a tethered cord. J Neurosurg 2006;105:391-5. [PubMed]
- Valentini LG, Selvaggio G, Erbetta A, et al. Occult spinal dysraphism: lessons learned by retrospective analysis of 149 surgical cases about natural history, surgical indications, urodynamic testing, and intraoperative neurophysiological monitoring. Childs Nerv Syst 2013;29:1657-69. [Crossref] [PubMed]
- Veenboer PW, Bosch JL, van Asbeck FW, et al. Paucity of evidence for urinary tract outcomes in closed spinal dysraphism: a systematic review. BJU Int 2013;112:1009-17. [PubMed]
- Metcalfe PD, Luerssen TG, King SJ, et al. Treatment of the occult tethered spinal cord for neuropathic bladder: results of sectioning the filum terminale. J Urol 2006;176:1826-9; discussion 1830.
- Ostling LR, Bierbrauer KS, Kuntz C. Outcome, reoperation, and complications in 99 consecutive children operated for tight or fatty filum. World Neurosurg 2012;77:187-91. [Crossref] [PubMed]
- Stavrinou P, Kunz M, Lehner M, et al. Children with tethered cord syndrome of different etiology benefit from microsurgery-a single institution experience. Childs Nerv Syst 2011;27:803-10. [Crossref] [PubMed]
- Poonia S, Graber S, Corbett Wilkinson C, et al. Outcome of hospital discharge on postoperative Day 1 following uncomplicated tethered spinal cord release. J Neurosurg Pediatr 2016;17:651-6. [Crossref] [PubMed]
- Jalai CM, Wang C, Marascalchi BJ, et al. Trends in the presentation, surgical treatment, and outcomes of tethered cord syndrome: A nationwide study from 2001 to 2010. J Clin Neurosci 2017;41:92-7. [Crossref] [PubMed]
- Levi B, Sugg KB, Lien SC, et al. Outcomes of tethered cord repair with a layered soft tissue closure. Ann Plast Surg 2013;70:74-8. [Crossref] [PubMed]
- Cornips EM, Vereijken IM, Beuls EA, et al. Clinical characteristics and surgical outcome in 25 cases of childhood tight filum syndrome. Eur J Paediatr Neurol 2012;16:103-17. [Crossref] [PubMed]
- Thuy M, Chaseling R, Fowler A. Spinal cord detethering procedures in children: a 5 year retrospective cohort study of the early post-operative course. J Clin Neurosci 2015;22:838-42. [Crossref] [PubMed]
- Strong MJ, Thompson EM, Roundy N, et al. Use of lumbar laminoplasty vs. laminotomy for transection of the filum terminale does not affect early complication rates or postoperative course. Childs Nerv Syst 2015;31:597-601. [Crossref] [PubMed]
- Yong RL, Habrock-Bach T, Vaughan M, et al. Symptomatic retethering of the spinal cord after section of a tight filum terminale. Neurosurgery 2011;68:1594-601; discussion 601-2. [Crossref] [PubMed]