Metastatic spine disease in lung cancer patients: national patterns of radiation and surgical care
Introduction
Lung cancer represents the leading cause of cancer-associated deaths worldwide and is among the most common primary malignancies to that metastasizes to the spine (1-4). Due to major strides in systemic therapies such as molecularly targeted agents and checkpoint inhibitors, there has been a marked increase in life expectancy of patients with metastatic lung cancer. The success of tyrosine kinase inhibitors in improving progression and overall survival rates for lung cancer serves as a quintessential example of this phenomenon (5). In light of increasing life expectancy of patients with metastatic spine disease, treatment must offer long-term disease control and a durable quality of life advantage (4,6-8). The increasing life expectancy for lung cancer patients underscores the need to consider outcomes on a lengthier time horizon (6).
The application of stereotaxis in radiation therapy (RT) has ushered in several new treatment approaches to tumors both within and outside of the central nervous system. Stereotactic radiosurgery (SRS) permits higher doses of radiation to be safely administered to tumors of the brain or spine while strategically minimizing exposure of healthy tissue. This is especially useful in treating traditionally radioresistant tumors and addressing pathology adjacent to critical neurological structures. SRS is frequently administered after spinal separation surgery—a surgical technique to decompress the spinal cord and reconstitute the thecal sac to create space between the spinal cord and tumor, allowing adequate SRS doses to be delivered to the tumor. When combined with separation surgery, SRS provides excellent long-term tumor control, while maximizing patient’s quality of life (9-11).
Given the evolving treatment of metastatic spine disease, we aimed to study long-term trends in treatment for the most common radioresistant tumor that metastasizes to the spine. In a national cohort of lung cancer patients with metastatic spine disease, the current study sought to: (I) evaluate national trends of radiation treatment and other systemic treatments; (II) determine the type and benefit of spinal surgery in this patient population; and (III) identify predictors of long-term survival in this population.
Methods
Study population
The National Cancer Database (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. This database aggregates an estimated 70% of newly diagnosed cancers in the United States through participation of over 1,500 CoC accredited facilities. Both academic and private member facilities across all regions of the United States contribute standardized, anonymous, patient-level clinical data regarding treatment administered at both the primary facility and at non-member facilities.
From 2004 to 2014, the NCDB was screened for patients with primary lung cancer who received RT to the spine. Spinal column radiation was used as a surrogate identifier of spinal metastases, as the NCDB otherwise indicates only presence or absence of bone metastases but does not specify the anatomical location. Due to their rarity compared to more conventional techniques, patients receiving brachytherapy, radioisotopes, or unknown radiation treatment were excluded.
Data collection
The data used in the study were derived from a de-identified NCDB file; all available clinical variables for the selected patients were included in the analysis. The study was exempt from Institutional Review Board approval and conforms to the provisions of the Helsinki Declaration. No patients’ identifiable health information was accessed, and data was maintained in a secured fashion.
Types of RT were categorized into the following three categories: external beam (EBRT), SRS, or particle-based therapy (neutrons or proton beam). EBRT included all energies of photon treatment, intensity-modulated RT (IMRT), and conformal or 3D RT. SRS included linear accelerator radiosurgery (Linac), Gamma Knife Surgery® (GKS; Elekta AB, Stockholm, Sweden), or SRS, not otherwise specified. Particle-based therapy included neutron or proton beam therapy.
With regard to surgical interventions, the type of procedure performed was coded according to the location of the primary site. For lung cancer, surgical procedures are as local tumor destruction, excision/resection of less than one lobe, lobectomy, or pneumonectomy, with options for laterality and lymph node dissection. The timing of surgery in days since the date of diagnosis was also recorded. Surgery on a non-primary site was coded with less specificity, as either resection of a regional site, distant site, distant lymph nodes, or a combination thereof. The anatomic location of the regional or distant site was not available. Both overall survival and duration of follow-up are measured in days since the date of diagnosis.
Statistical analysis
Descriptive statistics and changes over the 10-year span were provided. Specifically, rates of different radiation modalities, trends in treatment parameters, and patterns of surgical/chemotherapy treatments were assessed. One post-hoc assessment was performed to identify patients undergoing separation surgery, using the criteria of undergoing a surgical intervention on a distant metastasis followed by adjuvant SRS to the spine.
Non-parametric statistical tests were used, unless otherwise specified. The American College of Surgeons and the CoC have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data which are reported here. All analysis was performed in StataIC version 15 (College Station, TX: StataCorp LP).
Results
Demographics
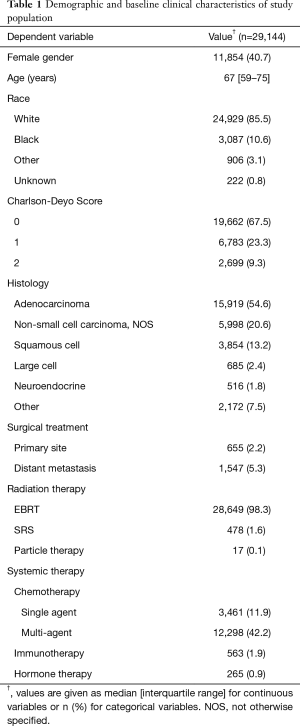
From an initial population of 1.285 M patients with lung cancer, 29,569 adult (i.e., age >17 years) patients who received RT to the spinal column were included. From this group, 425 additional patients who received a radiation treatment modality other than EBRT, SRS, or particle therapy were excluded, leaving a final study cohort of 29,144 patients. Characteristics of the study population are listed in Table 1.
Full table
Radiation trends and SRS
By definition, all patients received radiation treatment to the spine. The median treatment parameters were a dose of 30 Gy, administered over 10 treatments, lasting 15 days; the median delay from diagnosis to initiation of radiation was 18 days.
EBRT was the most frequent modality of spinal column RT across all years (Figure 1). Most EBRT patients received 10 treatments (IQR 10–14), with a median total dose of 30 Gy (IQR 27–35 Gy).
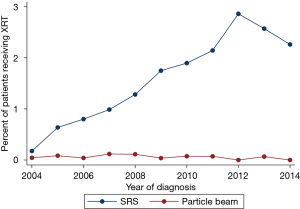
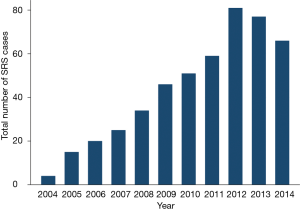
Of the patients receiving non-EBRT treatment, SRS was the most frequent radiation modality (Figure 2), and the number (and proportion) of SRS cases per year has steadily increased over time (Spearman’s rank correlation, P<0.01) as demonstrated in Figure 3. Only 17 patients received particle-based therapy. SRS was administered to a total of 478 patients, most often as a single treatment, with a median total dose of 24 Gy. Patients receiving SRS underwent a lesser number of treatments (Wilcoxon rank sum, P<0.01) with a smaller total dose (Wilcoxon rank sum, P<0.01), compared to the EBRT population.
Systemic and multimodal treatment
Chemotherapy was administered to 54.1% of patients and was most commonly part of a multi-agent regimen (78.0%). Immunotherapy and hormone therapy were given less frequently, to 563 (1.9%) and 265 (0.9%) of patients, respectively.
When treatment combinations were assessed, the most frequent regimen was external beam therapy alone (40.9% of patients). Thirty-seven percent of patients received multi-agent chemotherapy with EBRT, and 10.8% single-agent chemotherapy with EBRT.
Surgery
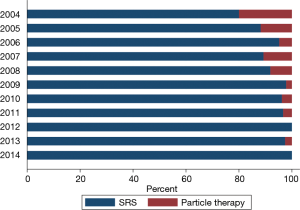
Within this cohort, 655 (2.2%) patients underwent a surgical procedure on the primary site of disease (lung), though this proportion trended downward (Figure 4; Spearman regression, P<0.01). Surgical resection of a non-primary site (i.e., any metastatic disease focus other than the lung) was performed in 1,547 (5.3%) patients, and the rate of non-primary-site surgery has increased over time (Figure 4; Spearman regression, P<0.01). The location of the non-primary disease site is not specified in the NCDB.
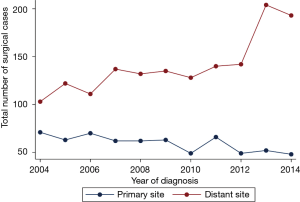
Among patients who received surgery of a distant metastatic site, 88.2% (1,364 patients) underwent radiation treatment after the surgical procedure. This was administered as EBRT in 97.8% of patients, and as SRS in the remaining 2.2% (30 patients).
In the post hoc analysis aimed at identifying possible separation surgery recipients, 30 patients were identified who underwent surgery of a distant metastasis followed by SRS to the spine. Given the lack of specific surgical information, including anatomic site, indication for surgery, and timing of radiation relative to surgery, no conclusions regarding the nature of spine surgery could be made.
Overall survival analysis
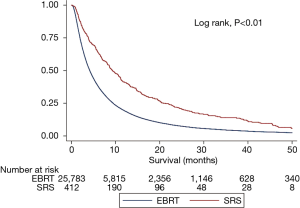
The median survival time for the study population was 4.21 months. Median survival was 6.24 months for patients receiving EBRT and 9.3 months for those receiving SRS (Figure 5). This difference was statistically significant using a univariate log rank test (P<0.01).
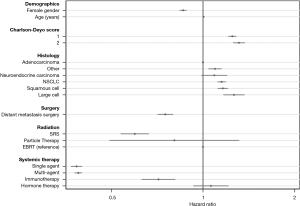
Next, using a priori selected available predictors of survival in metastatic lung cancer, we performed a multivariate Cox regression to assess the contribution of radiation modality to overall survival. The regression model included age, gender, Charlson-Deyo Score, histology, surgical treatment for the primary tumor and/or distant metastasis, chemotherapy, immunotherapy, hormone therapy, and modality of radiation treatment. Grade and clinical stage were not included in the model, as nearly all patients were stage 4 by virtue of the inclusion criteria, and grade was missing for the majority of patients. Hazard ratios for select variables are shown in Figure 6; coefficients for the various categorical histologies and grades can be seen in Table S1. After adjusting for other covariates in this Cox regression model, treatment with SRS was associated with a decreased rate of death when compared to EBRT (HR 0.59, P<0.01).
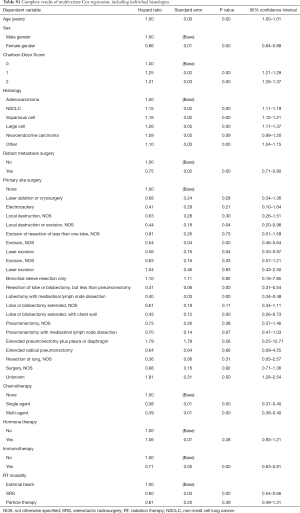
Full table
Discussion
As treatment of metastatic spine disease evolves with advance radiation and surgical techniques, establishing patterns in use and survival are needed. In the current study, we assessed national trends in spinal column radiation modality and the use of SRS throughout the entire country using the NCDB. From 2004 to 2014, the use of spinal SRS has increased. By surveying the landscape of treatment for spinal metastases from lung cancer, areas of future study are discussed below.
Radiation modality
EBRT remained the dominant modality of RT to the vertebral column. This may be due to modality preference or limited availability of SRS among treating radiation oncologists, as evidenced by the fact that from a survey of 1,600 members of the American Society for Radiation Oncology (ASTRO), only 64% reported having used stereotactic body RT as recently as 2010 (12).
There are several clinical scenarios that favor the use of conventional EBRT over SRS, however. EBRT is suitable especially for radiosensitive histologies where the augmented dose permitted by SRS is unnecessary, such as small cell lung cancer. In contrast, SRS is especially useful in treating radioresistant tumors or for patients who have previously failed EBRT, but can only be done to a maximum of 3 levels (13,14). Inability of the patient to tolerate immobilization precludes the use of frame-based SRS systems in favor of EBRT (if a frameless SRS system is not available), as well.
Although the rate of SRS increased over time, the continued widespread use of EBRT highlights the need to promote stereotactic treatment for radioresistant histologies, which requires constant education from larger centers and referral to centers with SRS capabilities. Opportunities for education are not limited to the oncology field, either; many neurosurgeons receive limited training in SRS techniques (15).
Surgical interventions
Approximately 5% of patients underwent surgical resection of a distant metastasis, and this proportion increased over time. Unfortunately, the NCDB does not provide the same procedural details for surgeries performed on distant metastases. Thus, no conclusions about spine surgery could be drawn and it would be inappropriate to do so. Considering the improved survival with metastatic spine disease and the benefit of separation surgery, we believe that the absence of surgical details is a significant weakness of this database, especially given the extensive RT data (10). Moreover, since separation surgery is a novel surgical approach predicated on post-operative SRS, it needs to be studied rigorously on a national level. In future iterations, we propose important surgical information be included, such as: Spinal Instability Neoplastic Score (SINS) (16), Epidural Spinal Cord Compression Score by Bilsky and colleagues (17), surgical indications, procedure/approach performed, and timing of surgery. With these surgery-specific variables collected, surgeons, radiation oncologists, and oncologists can work toward the optimal treatment plan for each patient. With the current data collection methods, this information cannot be determined.
In the previously described post-hoc analysis, very few patients met the criteria for separation surgery (i.e., surgery on a distant metastasis followed by RT to the spine). These criteria are inexact given the lack of surgical data. Even if only a subset of these patients truly underwent separation surgery, this would indicate that this surgical strategy is still uncommon. This may be a result of slow adoption of the technique, since separation surgery has been popularized mostly within the last decade primarily in leading specialized cancer centers (14,18,19).
Alternatively, low rates of surgery may be due to the method of data capture in the NCDB. The NCDB primarily captures new diagnoses of cancer, and hence late development of spinal metastases after initial diagnosis may not be captured if they occur in a very delayed fashion (20). Unfortunately, the coding of surgeries lacks the granularity to study the surgical management of distant metastases. This shortcoming demonstrates that opportunities for improvement exist in collecting data about surgical intervention in tandem with spinal SRS.
Survival analysis
Several factors were predictive of overall survival in this cohort. When survival curves for surgical patients were compared with RT type, SRS seemed to offer a statistically significant survival benefit. The indication for SRS versus EBRT may confound this relationship, hence a multivariable Cox regression was performed to adjust for other disease parameters which were available in the NCDB. In the multivariable model, RT modality was found to be an independent predictor of survival, where SRS was associated with longer OS. The significance of adjuvant RT modality was maintained (P<0.01).
Because SRS allows for greater biologic equivalent dose to the lesion of interest while minimizing radiation to health surrounding tissue, there is a biological basis for the possible survival benefit of SRS. In addition to the primary effect of SRS treatment, there is a theoretical synergistic effect of radiation treatment with newer systemic therapies, especially as use of targeted immunotherapies has become more widespread (21). Combined radiotherapy and systemic therapy may result in improved response of distant, non-radiated metastatic lesions, known as the abscopal effect (22). Treatment of brain metastases with concurrent radiation and newer targeted or immunotherapies is well tolerated (23). Trials evaluating this approach for spinal metastases are ongoing, although a phase I study of stereotactic radiotherapy with interleukin-2 for spinal melanoma metastases did show a promising response rate of 66% (24,25).
Many confounding factors which affect the decision to treat with EBRT or SRS are not captured in NCDB, such as a detailed measure of functional status, number and distribution of spine metastases. The lack of more detailed patient-level data meant that these other important prognostic variables could not be controlled for, so this survival difference should be rigorously assessed in a randomized controlled trial such as the recently completed Radiation Therapy Oncology Group (RTOG) 0631 study (26).
Limitations
There are several limitations to this study, some of which are inherent to the use of retrospective data from large, nationwide registries. Although we assessed overall survival using available predictors, histologic grade was missing for most patients, and functional status was not available. Hence, selection of different treatment options may have been guided by latent variables not assessed in this study.
Cohorts derived from a National database are affected by selection bias. There is limited anatomical information in the NCDB, so the cohort may have included patients with leptomeningeal or intramedullary metastatic disease, which is important because these patterns of disease are associated with very limited survival. This population is expected to be small given the relatively low prevalence of these patterns of metastasis compared to bone disease (27,28). Similarly, patients who underwent surgery for a spinal metastasis without subsequent RT would not have been captured here.
Trends in the overall use of SRS (including disease outside the spine) have been previously explored (29). Importantly, the nature and extent of surgical procedures on distant metastases were not available. Although other types of metastatic primary histologies frequently found in the spine could have been included as in other studies, we elected to study lung cancer to maintain a somewhat homogeneous disease population, systemic treatment options, and radiosensitivity (30). Given the high prevalence of lung cancer, selecting this disease was felt to provide the most homogeneity with the least degree of sample size sacrifice.
Lastly, functional outcomes cannot be assessed using the NCDB. Multi-modal therapies for patients with spinal metastatic disease are often administered for purposes of palliation rather than improved survival (19,31).
Future directions
This study demonstrates that the application of SRS to treatment of metastatic disease of the spine has steadily increased over time and that it may offer a survival advantage relative to traditional EBRT. With these new treatments and overall prolonged survival in lung cancer populations, it is feasible that a greater number of patients with metastatic spine disease will be suitable candidates for surgery. Currently, nationwide data regarding detailed surgical management of spinal metastatic disease is not available; many single-institution case series from specialized centers have aptly described procedural techniques and patient outcomes, but cannot offer perspective on how frequently this treatment is used more broadly (10,14). Future research must continue to monitor the adoption of procedures like separation surgery, so that barriers to adoption can be identified and addressed.
Conclusions
In the current national analysis over a 10-year span of patients with lung cancer and metastatic spine disease, several important trends emerged. The majority of RT is still administered as EBRT, yet the use of SRS has increased over time. Rates of surgery on distant metastases have steadily increased. This analysis suggests a possible survival advantage of SRS over EBRT, though this does not fully account for volume of disease, performance status, or other latent predictors not captured in the NCDB.
Acknowledgments
Funding: This work was supported by the National Cancer Institute of the National Institutes of Health (grant number T32CA106183 to PD Kelly).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was exempt from Institutional Review Board approval but conforms to the provisions of the Helsinki Declaration. Informed consent was waived as no patients’ identifiable health information was accessed, and data was maintained in a secured fashion.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543. [Crossref] [PubMed]
- Li C, Fang R, Sun Y, et al. Spectrum of oncogenic driver mutations in lung adenocarcinomas from East Asian never smokers. PloS One 2011;6:e28204. [Crossref] [PubMed]
- Harel R, Angelov L. Spine metastases: current treatments and future directions. Eur J Cancer 2010;46:2696-707. [Crossref] [PubMed]
- Dohzono S, Sasaoka R, Takamatsu K, et al. Overall survival and prognostic factors in patients with spinal metastases from lung cancer treated with and without epidermal growth factor receptor tyrosine kinase inhibitors. Int J Clin Oncol 2017;22:698-705. [Crossref] [PubMed]
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. [Crossref] [PubMed]
- Ho JC, Tang C, Deegan BJ, et al. The use of spine stereotactic radiosurgery for oligometastatic disease. J Neurosurg Spine 2016;25:239-47. [Crossref] [PubMed]
- Jensen G, Tang C, Hess KR, et al. Internal validation of the prognostic index for spine metastasis (PRISM) for stratifying survival in patients treated with spinal stereotactic radiosurgery. J Radiosurg SBRT 2017;5:25-34. [PubMed]
- Bilsky MH, Laufer I, Burch S. Shifting paradigms in the treatment of metastatic spine disease. Spine 2009;34:S101-7. [Crossref] [PubMed]
- Laufer I, Iorgulescu JB, Chapman T, et al. Local disease control for spinal metastases following “separation surgery” and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: outcome analysis in 186 patients. J Neurosurg Spine 2013;18:207-14. [Crossref] [PubMed]
- Zhang C, Wang G, Han X, et al. Comparison of the therapeutic effects of surgery combined with postoperative radiotherapy and standalone radiotherapy in treating spinal metastases of lung cancer. Clin Neurol Neurosurg 2016;141:38-42. [Crossref] [PubMed]
- Pan H, Simpson DR, Mell LK, et al. A survey of stereotactic body radiotherapy use in the United States. Cancer 2011;117:4566-72. [Crossref] [PubMed]
- Katsoulakis E, Kumar K, Laufer I, et al. Stereotactic body radiotherapy in the treatment of spinal metastases. Semin Radiat Oncol 2017;27:209-17. [Crossref] [PubMed]
- Laufer I, Rubin DG, Lis E, et al. The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist 2013;18:744-51. [Crossref] [PubMed]
- Yang I, Udawatta M, Prashant GN, et al. Commentary: Stereotactic Radiosurgery Training for Neurosurgery Residents: Results of a Survey of Residents, Attendings, and Program Directors by the American Association of Neurological Surgeons/Congress of Neurological Surgeons Section on Tumors. Neurosurgery 2019;8:E86-91. [Crossref] [PubMed]
- Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine 2010;35:E1221-9. [Crossref] [PubMed]
- Bilsky MH, Laufer I, Fourney DR, et al. Reliability analysis of the epidural spinal cord compression scale. J Neurosurg Spine 2010;13:324-8. [Crossref] [PubMed]
- Sciubba DM, Petteys RJ, Dekutoski MB, et al. Diagnosis and management of metastatic spine disease: a review. J Neurosurg Spine 2010;13:94-108. [Crossref] [PubMed]
- Moussazadeh N, Laufer I, Yamada Y, et al. Separation surgery for spinal metastases: effect of spinal radiosurgery on surgical treatment goals. Cancer Control 2014;21:168-74. [Crossref] [PubMed]
- Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683-90. [Crossref] [PubMed]
- Robin AM, Yamada Y, McLaughlin LA, et al. Stereotactic radiosurgery: the revolutionary advance in the treatment of spine metastases. Neurosurgery 2017;64:59-65. [Crossref] [PubMed]
- Bernstein MB, Krishnan S, Hodge JW, et al. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol 2016;13:516. [Crossref] [PubMed]
- Kroeze SG, Fritz C, Hoyer M, et al. Toxicity of concurrent stereotactic radiotherapy and targeted therapy or immunotherapy: a systematic review. Cancer Treat Rev 2017;53:25-37. [Crossref] [PubMed]
- Seung SK, Curti BD, Crittenden M, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2--tumor and immunological responses. Sci Transl Med 2012;4:137ra74. [Crossref] [PubMed]
- Caruso JP, Cohen-Inbar O, Bilsky MH, et al. Stereotactic radiosurgery and immunotherapy for metastatic spinal melanoma. Neurosurg Focus 2015;38:E6. [Crossref] [PubMed]
- Ryu S, Pugh SL, Gerszten PC, et al. RTOG 0631 phase 2/3 study of image guided stereotactic radiosurgery for localized (1-3) spine metastases: phase 2 results. Pract Radiat Oncol 2014;4:76-81. [Crossref] [PubMed]
- Jost G, Zimmerer S, Frank S, et al. Intradural spinal metastasis of renal cell cancer. Report of a case and review of 26 published cases. Acta Neurochir (Wien) 2009;151:815-21. [Crossref] [PubMed]
- Potti A, Abdel-Raheem M, Levitt R, et al. Intramedullary spinal cord metastases (ISCM) and non-small cell lung carcinoma (NSCLC): clinical patterns, diagnosis and therapeutic considerations. Lung Cancer 2001;31:319-23. [Crossref] [PubMed]
- McClelland S 3rd, Kim E, Passias PG, et al. Spinal stereotactic body radiotherapy in the United States: A decade-long nationwide analysis of patient demographics, practice patterns, and trends over time. J Clin Neurosci 2017;46:109-12. [Crossref] [PubMed]
- McClelland S, Kim E, Jaboin J, et al. Treatment of Spinal Metastases in the United States: Comparison of Conventional External Beam Radiation Therapy Versus Stereotactic Body Radiation Therapy Utilization Over a Multi-Year Timespan. Int J Radiat Oncol Biol Phys 2017;99:E93. [Crossref]
- Hariri O, Takayanagi A, Miulli DE, et al. Minimally invasive surgical techniques for management of painful metastatic and primary spinal tumors. Cureus 2017;9:e1114. [PubMed]