
Five-year clinical outcomes with endoscopic transforaminal outside-in foraminoplasty techniques for symptomatic degenerative conditions of the lumbar spine
Introduction
Endoscopic spinal decompression surgery has become feasible due to technological advancements and in Asian countries is often recommended as the preferred option for patients who have lumbar spinal stenosis (1). However, endoscopic spinal surgery remains outside the mainstream in the Americas and Europe, perhaps in part because its clinical superiority over other spinal decompression techniques has not been substantiated in controlled trials (2). Other factors contributing to the lower acceptance of endoscopic spinal surgery outside Asia may be cultural, training- and/or reimbursement-related (3).
The need for simplified, less burdensome, and more cost-effective spinal decompression surgeries is supported by the increased demand for these types of procedures with the aging baby-boomer population advancing into their retirement years. Most resource-strapped health care systems already struggle with the delivery of this spine care and are motivating stakeholders to come up with better-valued solutions both in terms of cost, and clinical outcomes. Patients increasingly also shy away from the aggressive treatment options of open spine surgery whose preoperative planning is often based in the traditional image-based decision-making (4). Staged outpatient endoscopic decompression of a painful degenerative lumbar motion segment causing claudication- and sciatica-type back and leg symptoms is a less disruptive alternative procedure compared to the myriad of traditional translaminar spinal decompression and fusion surgeries (5). In many cases, the patients’ subjective weakness, and intermittent claudication limiting walking endurance and other physical activities can be traced back to unilateral or single-level foraminal stenotic process as a frequent source of pain. Patients’ satisfaction with their clinical outcomes is highest when the final determination of the plan of care of their multilevel degenerative lumbar spine process is a shared decision between patient and provider based on a complex analysis of each patient’s painful pathoanatomy (6-8).
Long-term outcomes with the endoscopic transforaminal decompression procedure have not been well documented until recently when Yeung et al. published their 5-year follow up data with his widely publicized YESS™ inside-out foraminoplasty technique on a series of 86 patients (9). A similar data set does not exist with the outside-in technique which initially has been popularized by Hoogland et al. (10). and emerged over the years into an alternative to the inside-out endoscopic surgical protocol to treat lumbar herniated disc and spinal stenosis. While both techniques have similar goals of decompressing the neural elements, some procedural steps vary considerably and may impact long-term outcomes. In this study, the authors attempted to establish primary functional outcome measures, complication and reoperation rates in patients who underwent endoscopic transforaminal decompression for unrelenting sciatica-type low back and leg pain due to spinal stenosis with a minimum follow-up of 5 years.
Methods
Study groups & patient selection criteria
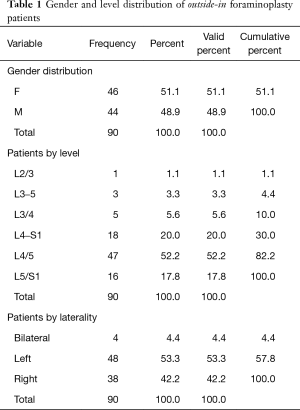
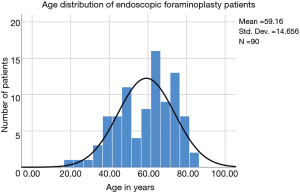
An outpatient endoscopic spinal surgery program for the treatment of lumbar herniated disc and spinal stenosis was established by the first author (Kai-Uwe Lewandrowski) In 2007 at the Center for Advanced Spine Care of Southern Arizona (11). Patients treated with the transforaminal outside-in decompression procedure popularized by Hoogland et al. (10). In this study, there were 90 patients (46 females, and 44 males) between the ages of 19 to 84 years and a mean of 59.16 years (Table 1; Figure 1). All patients were operated between 2012 to 2014 by the first- (Kai-Uwe Lewandrowski) and second (Nicholas A. Ransom) authors. Patients were selected from an extensive case log database maintained by the first author and included only in this study if 5-year follow-up data without gaps were available. Specifically, the following inclusion criteria were used:
- Symptomatic lumbar radiculopathy, dysesthesias, or decreased motor function;
- Magnetic resonance imaging (MRI) and computed tomography (CT) scans showing foraminal or lateral recess stenosis meeting criteria described below;
- Unrelenting pain in spite of failed physical therapy and transforaminal epidural steroid injections for at least 12 weeks.
The following exclusion criteria were employed:
- Severe central stenosis (less than 100 mm2) (12);
- Metastatic disease;
- Infection;
- Instability defined as the anterolateral translation of more than 3–5 mm or rotation of more than 10 to 15 degrees in the dynamic views (4).
Full table
Patients signed informed consent, and IRB approval was obtained (CEIFUS 106-19).
Radiologic evaluation of stenosis and classification
Advanced MRI and CT imaging studies were evaluated for foraminal and lateral recess stenosis. Extension/flexion radiographs were used to assess patients for spondylolisthesis. A stenotic process in the neuroforamen considered symptomatic was classified by location and severity using Lee’s radiographic classification systems and others (13-16). Lee’s foraminal zone stenosis classification within the neuroforamen divides it from medial to lateral into entry, middle, and exit zone (13). Hence, foraminal and lateral recess stenosis were stratified according to the primary offending pathology. Common problems causing neural element compression in the entry zone are due to hypertrophy of the superior articular process (SAP) of the facet joint. Osteophytic processes underneath the pars interarticularis were reported to cause stenosis in the mid- and in the exit zone. These can be caused by subluxation of a hypertrophied and unstable facet joint. The height of the intervertebral disc at the posterior intervertebral line and the height of the lumbar foramina on sagittal MRI images through the neuroforamina were measured considering the criteria published by Hasegawa et al. (14). On the basis of prior published correlative research between radiographic observations in patients with neurogenic claudication (13-16), parameters used to define spinal stenosis were posterior disc height of less than 3 mm, and neuroforaminal height less than 15 mm of on sagittal MRI images, and lateral recess height of less than 3 mm on axial MRI sequences. These indicators of reduced neuroforaminal volume were found to be radiographic prognosticators of neurogenic claudication symptoms in more than 80% of patients with spinal stenosis (14).
In addition to grading and recording the location and extent of foraminal stenosis on preoperative sagittal and axial MRI images, patients were also evaluated for extraforaminal stenosis on sagittal T1-weighted images. Lack of the regular interval of fat between the disc and nerve root was considered suggestive of extraforaminal stenosis. In this study, these radiographic criteria were used to stratify patients during the diagnostic workup of the lumbar level(s) believed to be causing the patient’s symptoms (see below) (16). The level frequency and laterality of surgery distribution are listed in Table 1 showing L4/5 (47/90 patients) and L5/S1 (16/90 patients) as the most common levels determined to require SED™ with foraminoplasty.
Workup & prognosticators of successful outcome
Patients suspected to suffer from neurogenic claudication symptoms were interviewed for the critical elements during the history taking and the physical examination. Typically, patients show a “normal” physical exam while resting. However, the symptomatic motor and sensory function abnormalities can often be easily provoked by asking patients to walk to the pain limit. The authors of this study included electromyography (EMG) and nerve conduction studies (NCS) as an adjunct to the diagnostic workup whenever they were available to help elucidate the clinical severity of symptoms and to assess the patients for the presence of peripheral neuropathy and other co-morbidities. If present, both could affect clinical results and increase the risk of postoperative complications (13). The EMG may be “normal” or “abnormal”, but the abnormal interpretation was considered by the authors as validation the patient’s subjective complaints (17). However, the authors did not routinely obtain electrodiagnostic studies since their usefulness is questionable because of their low specificity and sensitivity.
Several authors have serendipitously found that actual symptoms do not always correspond to advanced imaging (8,14). CT and MRI measurements were considered by the authors of this study as useful image-based criteria of lumbar spinal stenosis (18). Nevertheless, these guidelines needed to be corroborated with the additional use of preoperative diagnostic transforaminal epidural steroid injections (TESI) containing steroids and a local anesthetic (18). If a patient reported a 50% reduction in pain on the visual analog scale (VAS) for back and leg pain (19) in response to diagnostic injection, the TESI was considered diagnostic (18). The positive predictive value of preoperative diagnostic TESI for successful SED™ outcomes has been reported as high as 98.38% (18).
Surgical steps of the outside-in techniques
Patients suffering from symptomatic lumbar foraminal stenosis non-responsive to non-operative care were treated with the selective endoscopic discectomy (SED™)—a term coined and trademarked by Yeung in 2000—by employing the transforaminal approach with foraminoplasty in prone position under local anesthesia and sedation. Under continuous and direct videoendoscopic visualization, a modified outside-in technique was employed. After an initial foraminoplasty to accommodate the working cannula laterally at the facet joint with a good seal, the endoscopic instruments were then advanced into the lower portion of the neuroforamen. Kambin’s triangle bordered by the traversing and the exiting nerve root, as well as inferior pedicle was assessed and enlarged if necessary with an endoscopic 4.0 mm power drill (20). The exiting nerve root was retracted with the beveled tip of the working sheath to protect it and minimize irritation of its dorsal root ganglion (DRG) (11). Under continuous, and direct visualization, osteotomes, motorized drills, and Kerrison rongeurs were introduced through the inner 4.1 mm inner working channel of the spinal endoscope to perform the foraminoplasty. In some cases, the tip of the hypertrophied and upward migrated SAP had to be removed to expose the axilla of the exiting nerve root and the hidden zone of Macnab. An endoscopic osteotome was useful during these maneuvers. In patients with advanced disc degeneration and near complete vertical collapse of the spinal motion segment, an expansile foraminoplasty was performed by removal of additional bone from the inferior articular process (IAP) and the distal pedicle. The discectomy was commenced after the completion of the foraminoplasty without entering the intervertebral disc space with the endoscope or its working sheath.
Correlation of imaging to clinical presentation
Plain radiographs were carefully reviewed for any problems that could lead to the exclusion of the patient from the study. These problems included fractures due to trauma, and osteoporosis, near complete loss of disc height, spinal deformity greater than 30 degrees in the coronal plane, pars defects, facet hypertrophy and osteophytosis known as indicators of subclinical instability. The patients’ MRI and CT scans were evaluated for lateral recess and foraminal stenosis using the criteria listed above. Whenever available, CT myelography was considered the most accurate measure of any extradural causes of stenosis in the central and lateral canal. It was the mainstay of stenosis assessment in patients with suspected dynamic stenosis, postoperative leg pain, metallic implants, or any other contraindications to MRI scan. CT myelogram was explicitly ordered in patients whose lower extremity claudication symptoms could not be explained with plausible MRI findings (9). The preoperative protocol employed by the authors called for correlation of the imaging findings (21) with the patient’s response to diagnostic TESI (18). Only those patients whose preoperative workup was conclusive were scheduled for the transforaminal SED™.
Clinical follow-up & outcome analysis
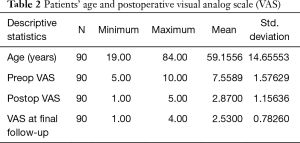
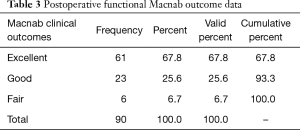
Primary clinical outcomes measures for patients who underwent the outside-in SED™ were the Macnab criteria at final follow-up 5 years postoperatively (22). The authors stratified patients towards Excellent and Good clinical outcomes for transforaminal SED™ under local anesthesia and sedation to validate the clinical protocols employed herein during the setup phase of the study. Patients with Fair and Poor postoperative Macnab outcomes were scrutinized in order of priority for inflammatory DRG irritation, recurrent stenosis, and instability, or the emergence of other pain generators within the index or other adjacent levels. Patients self-reported outcomes (PROMs) were obtained by soliciting a score on the VAS preoperatively (Preop VAS), within the immediate postoperative period (Postop VAS), and at final follow-up (Last F/U VAS) (9). Statistical tests employed in the outcome analysis of this study included two-tailed t-test, ANOVA testing, and two-way cross-tabulation statistics to measure any statistically significant association between variables using IBM SPSS Statistics software, version 25.0. Pearson Chi-square and Fisher’s Exact test were employed to assess the strength of association between variables statistically. The mean, range, and standard deviation (STD), and percentages of all nominal variables were calculated.
Results
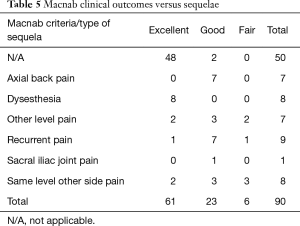
The age histogram of our study patients with a superimposed expected normal distribution curve was plotted and is shown in Figure 1. The average age was 59.16 years (STD 14.656 years) with the youngest patient being 19 years of age and the oldest patient 84 years, respectively. The mean follow-up was 71 months, ranging from 24 to 83 months. The outside-in SED™ was most commonly performed at the L4/5 level (52.2%) followed by unilateral two-level surgery L4–S1 (20%; Table 1). There was no statistical difference between left- (48/53.3%) versus right-sided (38/42.2%) surgery. Only 4 patients (4.4%) underwent bilateral SED™ at a single level (Table 1). Five years postoperatively, 67.8% (61/90) of patients reported Excellent, and another 25.6% (23/90) Good Macnab outcomes. Hence, 93.3% of all patients had Excellent and Good clinical outcomes according to Macnab 5 years postoperatively. A small minority of 6 patients (6.7%) reported Fair Macnab outcomes (Tables 2,3). The mean preoperative VAS was 7.5589. The mean postoperative VAS was 2.87 and 2.53 at last follow-up, respectively (P<0.0001).
Full table
Full table
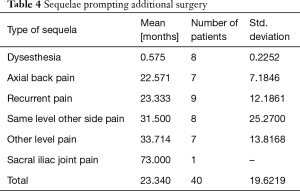
There were no study patients with significant complications related to approach, surgery or anesthesia. The vast majority of patients (58/90; 64.4% of the study population) did not require any additional interventional or surgical treatment following the index SED™. Postoperative dysesthesia due to irritation of the DRG occurred in 8 patients (8.9%) and was the most common benign postoperative sequelae (an unavoidable side effect of an otherwise expertly executed surgery; Tables 4-6). These patients were managed with activity modification, gabapentin or pregabalin, and transforaminal epidural steroid injections. Most patient’s DRG irritation symptoms resolved with these supportive care measures within 2 to 3 weeks. No other complications were observed in the immediate 90-day postoperative period or after that.
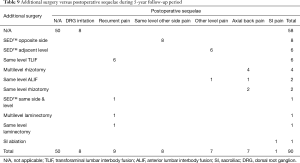
Additional surgeries were done on average 23.34 months from the index SED™ in 32 (35.6%) of the 90 patients (Tables 4-6). There was variation in the average time elapsed from the index SED™ procedure to additional intervention (Tables 4-6). Development of axial back pain after SED™ prompted additional surgery at an average of 22.57 months postoperatively (7/90; 7.8%). This was followed by recurrence of familiar pain from the same surgical level at an average of 23.33 months (9/90; 10%), development of sciatica type back and leg pain on the opposite side but from the same surgical level at an average of 31.5 months (8/90; 8.9%), and development of sciatica type back and leg pain from a different adjacent level at an average of 33.71 months (7/90; 7.8%), respectively. Recurrence of familiar pain was due to natural progression of the degenerative disease process causing vertical collapse and nerve root entrapment. There were no extruded disc herniations in follow-up. One additional patient underwent ablation of his painful sacroiliac (SI) joint at 73 months following the index SED™ (1/90; 1.1%).
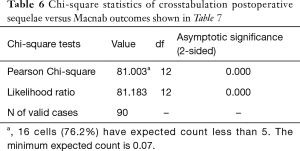
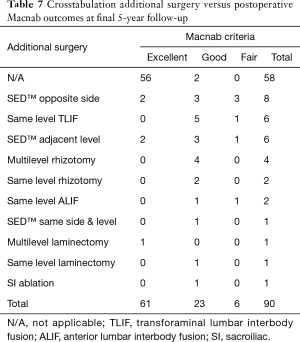
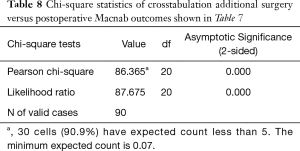
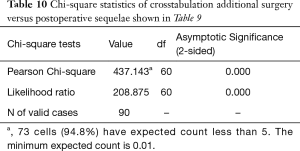
Cross-tabulation of long-term outcomes versus additional intervention (Tables 4-7) showed that patients, who initially rated their VAS scores and Macnab outcomes favorably, also did so at final follow-up and did not require any additional intervention or surgery throughout their total follow-up period. The 50 patients without any need for additional intervention and the 8 patients with DRG irritation, who were successfully treated with TESI and did not require any additional surgery throughout the follow up period, totaled a 64.4% (58/90; Table 7) portion of the entire patient population who did not receive any additional surgery following the index SED™. Moreover, functional outcomes reported at long-term 5-year follow-up were not affected by the need for additional surgeries (P<0.0001) with the majority of the additional surgeries (26/32; 81.25%; Table 7) being performed on patients who ultimately rated their outcome as Excellent and Good. Only 5 of the 61 patients (5.56% of total study population n=90) with Excellent results opted for more surgery with 2 patients undergoing SED™ on the opposite side at the same level, another 2 patients undergoing same level TLIF, and 1 patient being treated with a laminectomy (Table 7). The latter 3 patients underwent surgery for the progression of the degenerative disease process. Another 6 patients who reported Fair long-term outcomes developed pain from adjacent segment disease (2 patients), recurrent pain from the same level (1 patient), and pain from the opposite side of the same surgical level (3 patients). The majority of patients (21/90; 23.3%) who opted for additional surgery within the minimum 5-year follow-up period rated their long-term 5-year Macnab outcomes as Good (Table 7). The specific procedures were cross-tabulated by outcome (Tables 7,8), and by the problem that prompted the additional follow-up surgery following the index SED™ (Tables 9,10). While most of the follow-up surgeries following SED™ were additional endoscopic decompressions and rhizotomies (24/32; 75%) and were non-fusion procedures, only 8 patients of the whole study series of 90 (8.9%) underwent a fusion after index SED™ within the minimum 5-year follow-up period. One patient opted for an open laminectomy (1.1%).
Full table
Full table
Full table
Full table
Full table
Full table
Full table
Discussion
This study’s primary outcome measures showed favorable Excellent and Good long-term clinical results with the endoscopic transforaminal outside-in decompression procedure in the 93.3% of patients. This 5-year data set was intended for comparison to a recently published study of similar design on the long-term outcomes with the endoscopic inside-out technique (9). The authors employed a conceptually different transforaminal decompression methodology than used in the recently published long-term outcome study (9), which employed the inside-out YESS™ technique (8). The endoscopic decompression technique employed in this study is a modification of the original outside-in method popularized by Hoogland et al. (10). Instead of using percutaneous cannulated reamers or trephines over a guidewire as recently described (23), the initial foraminoplasty on patients of this study was performed under direct visualization with a motorized power drill that was passed through the inner working channel of the spinal endoscope, thus, lowering the risk of forceful injury to the facet joint complex or the nerve roots which has been observed in some patients with the original TESSYS® technique (24). The sharpened trephines’ cutting teeth can cause nerve root traction injury by catching foraminal ligaments or adhesions frequently seen in the presence of facet joint cyst that can be attached to the nerve roots (24). In the first author’s 12-year operative experience of endoscopic spine surgery, early-on elimination of handheld sharp-teethed cannulated trephines and reamers used over a guidewire in a non-visualized manner decreased the incidence of postoperative irritation of the DRG substantially and prompted the first author’s modification of the original TESSYS® technique to be more consistent with the surgically more refined contemporary Maxmore® technique. The first author’s advancements of the outside-in transforaminal technique culminated in the development of an FDA-approved endoscopic spinal decompression system (25,26) which was employed in the 90 study patients.
Aging of the lumbar spinal motion segment eventually produces instability and hypermobility. Corresponding MRI findings include thickening of the ligamentum flavum, the soft tissues in the foramen, and hypertrophy of the facet joints, particularly at the SAP (4). Spinal stenosis related symptoms may begin with subjective numbness, and periodic weakness due to decreased walking endurance. Claudication symptoms may gradually progress and increase the cumulative disability to a point, where the patient is no longer able to gain control of them by accommodation or treatment with supportive care measures. In spite of having these symptoms, patients are often caught up in the controversy on appropriate surgical indications and the best timing of surgical intervention for lumbar foraminal stenosis that does not respond favorably to standard non-operative and supportive care measures. Therefore, the authors employed patient selection protocols in this study that have been thoroughly vetted through a rigorous peer-review process and have been published in several high-ranking journals (11,18,21,27). The authors’ use of specific advanced CT or MRI imaging prognosticators associated with favorable clinical outcomes following the outside-in endoscopic transforaminal decompression procedure for foraminal stenosis let to the inclusion of patients suffering from sciatica-type low back and leg pain which by traditional imaging criteria were either “not bad enough”, or “too young”, or “too old”—the misfits—to be considered for conventional open surgical decompression. A recently published analysis by the first author on 1,839 spinal endoscopy patients found a 30% false negative rate difference between radiologist lumbar MRI reporting of foraminal stenosis and surgeon grading (21). In the United States, this diagnostic gap creates a continuity of care problem since the MRI report dominates the medical necessity determination for surgery imposed by many payers. Consequently, underreporting of clinically relevant MRI findings often leads to undertreatment of symptomatic lumbar foraminal stenosis entrapping many patients in ineffective and repetitive referral cycles to physical therapy and pain management. Subsequent progression of the degenerative disease process in untreated patients to more advanced stages may further exacerbate the opioid abuse crisis (28), and it may contribute to mounting disability and direct (cost of non-surgical treatments) and indirect cost (i.e., lost wages) to patients and their families who are trying to cope with the unintended consequences of poorly managed lumbar spinal stenosis symptoms and its resultant physical and mental health as well as social decline (29). Ultimately, such delays may create the need for more complex and costly definitive treatments that may come at a much higher risk to the patient.
Therefore, this study was motivated by the need to validate the early treatment of symptomatic foraminal stenosis by researching what happens to endoscopic foraminoplasty patients in the long-run. Other important associated questions revolved around what type of planned and unplanned surgical and non-surgical aftercare, if any, was necessary to treat any shortcomings of the procedure and what was the longevity of the treatment effect as defined by the ultimate goal of avoiding spinal fusion five years after the initial endoscopic index procedure? In short, the authors attempted to define the inherent comparative societal value of the healthcare delivered via the outside-in endoscopic transforaminal decompression—a discussion that is highly timely in the context of reforming healthcare from population-based management guidelines to personalized care plans.
Just reporting the 5-year Excellent (67.8%) and Good (25.6%) long-term Macnab standardized outcome criteria and statistically significant VAS reductions (P<0.0001; Table 3) would be an oversimplification of what goes into maintaining favorable long-term outcomes with the transforaminal endoscopic outside-in decompression procedure. While nearly two-thirds of the study population (64.4%) never required any additional intervention, approximately one-third (35.6%) had some unintended aftercare postoperatively. Surprisingly, this was not always associated with Fair outcomes (Tables 7-10). The majority of unintended aftercare was delivered to patients with Excellent and Good outcomes (17 patients). Therefore, the authors of this study conclude that long-term outcomes with endoscopic transforaminal foraminoplasty are at a minimum similar to outcomes reported with microdiscectomy or laminectomy (17,30,31). For example, there were no extruded disc- or simple contained reherniations without associated bony stenosis within the immediate 90-day postoperative study period, at 2-, or 5-year follow-up. The published recurrence rate is around 5% (32). Only 9 patients (10%) reported a recurrence of the same familiar pain stemming from the surgical index level within the 5-year follow-up period. A recent study on 1,856 patients reported the long-term cumulative incidences of reoperation rates after open discectomy, laminectomy, percutaneous endoscopic lumbar discectomy, and fusion as 4% at 1 year, 6% at 2 years, 8% at 3 years, 11% at 5 years and 16% at 10 years (30). The authors of that study found 10-year cumulative reoperation risk of 16%, 14%, 16% and 10% after open discectomy, laminectomy, percutaneous endoscopic lumbar discectomy, and fusion, respectively, with no statistically significant difference in reoperation rates when cross-tabulated by the type of surgical technique during the index procedure. However, the choice of the latter significantly impacted the choice of technique used during the revision surgery. The authors of that large multicenter study found that the preferred reoperation surgical technique was another open discectomy in 80% of open discectomy patients and 81% of patients after percutaneous endoscopic lumbar discectomy. In comparison, our reoperation rate with fusion was only 8.9%. Another study on 214 patients found lower 10-year reoperation rates (15.2% vs. 3.7%) with less aggressive unilateral hemilaminectomies versus complete laminectomies (31). Compared to established complication rates (27) with open lumbar spine surgery or other forms of translaminar or transforaminal minimally invasive spinal surgery, there were no complications in this series of 90 patients. Uncommon postoperative problems after endoscopic spinal surgery include dural tears, infections, wrong level surgeries, foot drop, pedicle-, and facet fracture, or pulmonary emboli. None of these were encountered in our patient group. Nearly all of the unintended aftercare was due to benign transitory irritation of the DRG at the operational level, which was treated successfully with TESI.
In our study, common scenarios for additional surgery within the 5-year follow-up period following the index SED™ were new-onset of different pain on the opposite side of the same index level in patients who underwent a unilateral index SED™ decompression. Older patients with multilevel disease reported new-onset of unfamiliar pain stemming from a different level. The presence of some patients with recurrence of unfamiliar pain from the same SED™ index level within the 5-year follow-up period indicates that patients may have multiple pain generators within one motion segment or may develop new ones in follow-up due to the progression of the degenerative disease process that can influence clinical outcomes. These observations underline the importance of developing a personalized treatment plan for each patient to maximize the benefit from the endoscopic decompression procedure instead of basing the surgical plan solely on advanced imaging studies. The endoscopic spine surgeon should take advantage of the ability to directly visualize and assess the painful pathoanatomy in the sedate yet awake patient. This is best done by correlating these intraoperative observations with preoperative imaging studies. Mechanical compression of the exiting or traversing nerve root created by a herniated disc or bony obstruction of the neuroforamen may not be the only pain generator within a symptomatic lumbar motion segment (8,9). Painful leaking annular tears within the central portion of a contained disc herniation under the dural sack, entrapped extruded disc herniation within the annulus, painful nerve root tethering by foraminal ligaments, or facet cysts in the lateral recess and neuroforamen are common problems that are easily missed on preoperative MRI scanning. They could be missed during the SED™ unless the surgeon carefully uses all diagnostic tools at his or her disposal (6-8,21). Correlative pre- and intraoperative evocative and analgesic diagnostic injections, epidurography, and discography are very useful tools during the intraoperative diagnostic process.
To not interfere with this intraoperative diagnostic workup, the initial foraminoplasty with power drills was intended to merely create a safe space for the working sheath to be placed. The beveled working cannula was routinely positioned by facing the lateral aspect of the facet joint complex to obtain a good seal at the beginning of the surgery while maintaining good irrigation pressure and visualization without infusing the paraspinal tissues with excessive amounts of irrigation fluid before advancing into Kambin’s triangle (20). Moreover, the authors considered this a way of assuring that the intraforaminal anatomy is not distorted or destroyed, as is possible during the fluoroscopically guided drilling or reaming called for by the TESSYS® method (24), before such real-time intraoperative evocative evaluation of pain generators in the awake patient has been completed. Therefore, the initial foraminoplasty with power drills was intended to merely create a safe space for the working sheath to be placed in by facing the lateral aspect of the facet joint complex at the beginning of the surgery before advancing into Kambin’s triangle (20). The authors considered this a way of assuring that the intraforaminal anatomy is not distorted or destroyed, as is possible during the fluoroscopically guided drilling or reaming called for by the TESSYS® method (24), before such real-time intraoperative evocative evaluation of pain generators in the awake patient has been completed.
This retrospective study had some limitations. This study was done on a small group of 90 patients between two surgeons whose follow-up data was available up to 5 years postoperatively without gaps. Moreover, affective (unconscious emotional reaction) and cognitive (distortions of thinking) biases in the clinical diagnostic and surgical decision-making process by the authors (33,34). Hindsight or outcome bias, are virtually unavoidable in retrospective studies and are well known cognitive biases. The cumulative knowledge of the clinical outcomes by the surgeon during a retrospective study has been recognized to inflate the predictability of an event after it occurred (35-38). Hindsight cognitive biases may have been less relevant since the individual patient-specific pain generators ascertained during awake intraoperative evaluation under local anesthesia were not known throughout the 7-year study period. Intuition bias (38) may have played a role in patient selection for surgery after the initial learning curve.
While the distinction between the outside-in and the inside-out techniques for the foraminoplasty discussion may seem academic on the surface, to those who practice endoscopic spine surgery at the highest level, it is far from it. This long-term clinical outcome study on the utility of the outside-in transforaminal decompression provides an additional data set for comparison to another recently published 5-year follow-up study of similar design (9) and provides an opportunity for an illustrative discussion and study of the many distinct procedural steps between these two techniques, their pros and cons, and how the skilled endoscopic spine surgeon may apply them to benefit of her or his patients by avoiding pitfalls and by capitalizing on advantages that ultimately may play out in short- and long-term follow-up. Most importantly though both studies support the concepts of staged surgical management of lumbar foraminal and lateral recess stenosis painful at the time when the care is delivered with only one-third of patients requiring additional interventional and some surgical treatment at 5-year minimum follow-up. Ultimately, only a small portion of patients—8.9% in this study—may require a fusion to continue to manage their symptoms.
Conclusions
Patients with symptomatic foraminal stenosis can be treated favorably with early outside-in transforaminal endoscopic decompression. Medical necessity and coverage guidelines for translaminar open lumbar spinal surgery often recommend delaying surgical decompression for sciatica-type back and leg pain due to foraminal and lateral recess stenosis because of a significant burden to the patient, high cost, and the propensity to develop post-laminectomy instability and the need for fusion in the future. The transforaminal endoscopic decompression procedure provides direct access to the stenotic neuroforamen and has low propensity to destabilize the lumbar spinal motion segment. It facilitates the early and effective intervention of debilitating painful degenerative conditions of the lumbar spine by recognizing and treating the predominant pain generator at the time when the care is delivered. This ambulatory spine care can be safely delivered at a lower cost in an outpatient surgery center. Age-related progression of the degenerative disease process may produce new-onset or recurrent symptoms in the long-term, which often can be managed with additional outpatient endoscopic procedures. Fusions are rarely required. This knowledge will most likely become mainstream once the concepts of endoscopic interventional spine surgery illustrated in this study are validated with high-grade clinical studies.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no direct (employment, stock ownership, grants, patents), or indirect conflicts of interest (honoraria, consultancies to sponsoring organizations, mutual fund ownership, paid expert testimony). The authors are not currently affiliated with or under any consulting agreement with any vendor that the clinical research data conclusion could directly enrich. This manuscript is not meant for or intended to endorse any products or push any other agenda other than the associated clinical outcomes with lumbar endoscopic foraminoplasty. The motive for compiling this clinically relevant information is by no means created and/or correlated to directly enrich anyone due to its publication. This publication was intended to substantiate contemporary endoscopic spinal surgery concepts to facilitate technology advancements.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. IRB approval was obtained (CEIFUS 106-19), and all patients signed informed consent for publication of this manuscript and any accompanying images.
References
- Choi G. Small is Beautiful, Less is Better. Neurospine 2019;16:3. [Crossref] [PubMed]
- Butler AJ, Alam M, Wiley K, et al. Endoscopic Lumbar Surgery: The State of the Art in 2019. Neurospine 2019;16:15-23. [Crossref] [PubMed]
- Kim JS. A bibliometric study in the field of percutaneous full-endoscopic spine surgery since 1997. Annual NASS Meeting 2018, Los Angeles. After Hours: Endoscopic Spine Surgery: Current Trends & Evidence of Spinal Endoscopic Procedures, Thursday, September 27, 2018. Available online: https://exhibit.nassannualmeeting.org/AM2018/Public/SpeakerDetails.aspx?FromPage=Sessions.aspx&ContactID=12065
- Rauschning W. Normal and pathologic anatomy of the lumbar root canals. Spine (Phila Pa 1976) 1987;12:1008-19. [Crossref] [PubMed]
- Yeung AT. Endoscopic Decompression, Foraminalplasty and Dorsal Rhizotomy for Foraminal Stenosis and Lumbar Spondylosis: A Hybrid Procedure in Lieu of Fusion. J Neurol Disord 2016;4:322. [Crossref]
- Yeung A, Yeung CA. Endoscopic Identification and Treating the Pain Generators in the Lumbar Spine that Escape Detection by Traditional Imaging Studies. J Spine 2017;6:369.
- Yeung AT, Gore S. In-vivo Endoscopic Visualization of Patho-anatomy in Symptomatic Degenerative Conditions of the Lumbar Spine II: Intradiscal, Foraminal, and Central Canal Decompression. Surg Technol Int 2011;21:299-319. [PubMed]
- Yeung AT. The Yeung Percutaneous Endoscopic Lumbar Decompressive Technique (YESSTM). J Spine 2018;7:408. [Crossref]
- Yeung A, Roberts A, Zhu L, et al. Treatment of Soft Tissue and Bony Spinal Stenosis by a Visualized Endoscopic Transforaminal Technique Under Local Anesthesia. Neurospine 2019;16:52-62. [Crossref] [PubMed]
- Hoogland T, Schubert M, Miklitz B, et al. Transforaminal posterolateral endoscopic discectomy with or without the combination of a low-dose chymopapain: a prospective randomized study in 280 consecutive cases. Spine (Phila Pa 1976) 2006;31:E890-7. [Crossref] [PubMed]
- Lewandrowski KU. “Outside-in” technique, clinical results, and indications with transforaminal lumbar endoscopic surgery: a retrospective study on 220 patients on applied radiographic classification of foraminal spinal stenosis. Int J Spine Surg 2014. [Crossref] [PubMed]
- Sengupta DK, Herkowitz HN. Lumbar spinal stenosis. Treatment strategies and indications for surgery. Orthop Clin North Am 2003;34:281-95. [Crossref] [PubMed]
- Lee CK, Rauschning W, Glenn W. Lateral lumbar spinal canal stenosis: classification, pathologic anatomy and surgical decompression. Spine (Phila Pa 1976) 1988;13:313-20. [Crossref] [PubMed]
- Hasegawa T, An HS, Haughton VM, et al. Lumbar foraminal stenosis: critical heights of the intervertebral discs and foramina. A cryomicrotome study in cadavera. J Bone Joint Surg Am 1995;77:32-8. [Crossref] [PubMed]
- Kirkaldy-Willis WH, Wedge JH, Yong-Hing K, et al. Lumbar spinal nerve lateral entrapment. Clin Orthop Relat Res 1982.171-8. [PubMed]
- Jenis LG, An HS. Spine update: lumbar foraminal stenosis. Spine (Phila Pa 1976) 2000;25:389-94. [Crossref] [PubMed]
- Yeung A. Failed Back Surgery Syndrome: Endoscopic Documentation of Common Causes by visualization of Painful Patho-anatomy in the hidden zone of the axilla containing the Dorsal Root Ganglion and Salvage treatment of Neuropathic pain with DRG neuromodulation. SF J Neuro Sci 2017;1:1.
- Lewandrowski KU. Successful outcome after outpatient transforaminal decompression for lumbar foraminal and lateral recess stenosis: The positive predictive value of diagnostic epidural steroid injection. Clin Neurol Neurosurg 2018;173:38-45. [Crossref] [PubMed]
- Botwin KP, Gruber RD, Bouchlas CG, et al. Fluoroscopically guided lumbar transforaminal epidural steroid injections in degenerative lumbar stenosis. Am J Phys Med Rehabil 2002;81:898-905. [Crossref] [PubMed]
- Kambin P, Casey K, O’Brien E, et al. Transforaminal arthroscopic decompression of lateral recess stenosis. J Neurosurg 1996;84:462-7. [Crossref] [PubMed]
- Lewandrowski KU. Retrospective analysis of accuracy and positive predictive value of preoperative lumbar MRI grading after successful outcome following outpatient endoscopic decompression for lumbar foraminal and lateral recess stenosis. Clin Neurol Neurosurg 2019;179:74-80. [Crossref] [PubMed]
- Macnab I. Negative disc exploration: An analysis of the causes of nerve-root involvement in sixty-eight patients. J Bone Joint Surg Am 1971;53:891-903. [Crossref] [PubMed]
- Jasper GP, Francisco GM, Aghion D, et al. Technical considerations in transforaminal endoscopic discectomy with foraminoplasty for the treatment of spondylolisthesis: Case report. Clin Neurol Neurosurg. 2014;119:84-7. [Crossref] [PubMed]
- Li ZZ, Hou SX, Shang WL, et al. Modified Percutaneous Lumbar Foraminoplasty and Percutaneous Endoscopic Lumbar Discectomy: Instrument Design, Technique Notes, and 5 Years Follow-up. Pain Physician 2017;20:E85-98. [Crossref] [PubMed]
- FDA 510k Number K150428, Device Name Integra (R) Jarit (R) Kerrison Rongeurs, Integra (R) Ruggles (TM)-Redmons (TM) Kerrison Rongeurs, Integra (R) Miltex (R) Kerrison Rongeurs, Integra (R) Meisterhand (R) Kerrison Rongeurs. Date Received: 02/19/2015, INTEGRA LIFESCIENCES CORP. 311 ENTERPRISE DR. Plainsboro, NJ 08536 Decision Date: 04/16/2015, Decision: Substantially Equivalent (SESE). Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K150428
- FDA 510(K) Number K121261, Device Classification Name Arthroscope, Device Name ASAP MUITISCOPE ASAP ENDOSCOPIC PRODUCTS GMBH, AMSTEL 320-I, Amsterdam, NL 1017ap, Date Received: 04/26/2012, Decision Date: 01/08/2013, Decision: Substantially Equivalent (SESE). Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K121261
- Lewandrowski KU. Incidence, Management, and Cost of Complications After Transforaminal Endoscopic Decompression Surgery for Lumbar Foraminal and Lateral Recess Stenosis: A Value Proposition for Outpatient Ambulatory Surgery. Int J Spine Surg 2019;13:53-67. [Crossref] [PubMed]
- Cheatle MD. Facing the challenge of pain management and opioid misuse, abuse, and opioid-related fatalities. Expert Rev Clin Pharmacol 2016;9:751-4. [Crossref] [PubMed]
- Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ 2003;81:646-56. [PubMed]
- Kim CH, Chung CK, Choi Y, et al. The Long-Term Reoperation Rate Following Surgery for Lumbar Herniated Intervertebral Disc Disease: A Nationwide Sample Cohort Study with A 10-Year Follow-Up. Spine (Phila Pa 1976) 2019. [Crossref] [PubMed]
- Pietrantonio A, Trungu S, Famà I, et al. Long-term clinical outcomes after bilateral laminotomy or total laminectomy for lumbar spinal stenosis: a single-institution experience. Neurosurg Focus 2019;46:E2. [Crossref] [PubMed]
- Asch HL, Lewis PJ, Moreland DB, et al. Prospective multiple outcomes study of outpatient lumbar microdiscectomy: should 75 to 80% success rates be the norm? J Neurosurg 2002;96:34-44. [PubMed]
- Bakeman R, Quera V, McArthur D, et al. Detecting sequential patterns and determining their reliability with fallible observers. Psychol Methods 1997;2:357-70. [Crossref]
- Sibbald M, Cavalcanti RB. The biasing effect of clinical history on physical examination diagnostic accuracy. Med Educ 2011;45:827-34. [Crossref] [PubMed]
- Zwaan L, Monteiro S, Sherbino J, et al. Is bias in the eye of the beholder? A vignette study to assess recognition of cognitive biases in clinical case workups. BMJ Qual Saf 2017;26:104-10. [Crossref] [PubMed]
- Henriksen K, Kaplan H. Hindsight bias, outcome knowledge and adaptive learning. Qual Saf Health Care 2003;12:ii46-50. [Crossref] [PubMed]
- Hugh TB, Tracy GD. Hindsight bias in medicolegal expert reports. Med J Aust 2002;176:277-8. [Crossref] [PubMed]
- Wübken M, Oswald J, Schneider A. Dealing with diagnostic uncertainty in general practice. Z Evid Fortbild Qual Gesundhwes 2013;107:632-7. [PubMed]


