
Decreased estimated blood loss in lateral trans-psoas versus anterior approach to lumbar interbody fusion for degenerative spondylolisthesis
Introduction
Degenerative lumbar spondylosthesis is characterized by progressive instability of the lumbar spine and most commonly occurs at the L4–5 segment (1). Surgical decompression and fusion with or without instrumentation is an effective treatment for persistent symptoms refractory to conservative management (2-5). Whether decompression alone or decompression with fusion is superior remains controversial (6-11). Several approaches for degenerative spondylolisthesis have been described (3,12,13); however, the best approach remains debatable (14).
The eXtreme lateral trans-psoas approach (XLIF) provides an alternative method for accessing the anterior lumbar spine, with the goal of maximizing graft surface area while minimizing exposure-related complications that can occur with a direct anterior approach (15). Lumbar fusion through the XLIF approach is associated with low estimated blood loss, improvement in post-operative patient reported outcomes, and low complication rates, but is also associated with exposure-related sensory and motor changes in the ipsilateral lower extremity, particularly at L4–5 where the lumbar plexus may be more anterior and the iliac crest may limit direct lateral access (16,17). Anterior and XLIF trans-psoas approaches for single level fusions at any lumbar segment yield similar complication rates, radiographic and early clinical outcomes (18-20). There is, however, a paucity of literature directly comparing anterior lumbar interbody fusion (ALIF) with XLIF for degenerative spondylolisthesis. To better inform the choice of surgical approach for treatment of degenerative spondylolisthesis, the specific complications and outcomes associated with these approaches need to be delineated (21).
Therefore, the purpose of this study was to compare the peri-operative outcomes, complications, and patient-reported outcomes of patients with degenerative spondylolisthesis treated at a single level with XLIF versus ALIF.
Methods
This retrospective, single-institution study was approved by the Institutional Review Board at our academic institution (IRB # 7935), and consent was waived by the IRB for the retrospective chart review. All anterior and anterolateral fusion cases from 4 fellowship-trained orthopaedic and neurosurgeons were identified using CPT code 22558 over the period from 2008–2012. Overall, 1,065 instances of the particular CPT code occurred. To create as homogeneous of a cohort as possible, we included only patients undergoing lumbar interbody fusion via an ALIF or XLIF approach at the single L4–5 level for a diagnosis of degenerative spondylolisthesis. Patients were required to have at least 30 days of follow up for complications. ALIF or XLIF was performed at discretion of operating surgeon. A vascular access surgeon was used for anterior approaches. For XLIF, neuromonitoring was used during cage insertion. Screws were placed percutaneously and utilized neuromonitoring. Patient demographics were recorded from the electronic medical record (EMR). Differences in peri-operative data (estimated blood loss, operative time, and adjunct procedures or additional implants), 30-day complications (infection, DVT/PE, stroke, weakness/paresthesias, leg pain, and ileus), and overall re-operation rates at L4–5 level were also identified via the EMR. Fusion was assessed on lateral lumbar films (22) (grade I, fused with remodeling and trabeculae; grade II, graft intact, not fully remodeled and incorporated though but with no lucencies above or below; grade III, graft intact but a definite lucency at the top or bottom of the graft; grade IV, definitely not fused with resorption of bone graft and with collapse). Subsidence >2 mm was documented as previously described (23). Disc height was calculated on lateral radiographs as the average of the anterior and posterior disc height normalized against the mid-sagittal diameter of the L4 vertebral body as previously described (24). Pre- and post-operative L1–S1 Cobb angle and disc height were calculated from radiographs. Spondylolisthesis was characterized by Meyerding grade (25). Lumbar stenosis was characterized on pre-operative MRI. At pre- and post-operative clinic visits, patients were asked to complete the following outcomes tools: Numerical Rating Scales (NRS) for back and leg pain and the Oswestry Disability Index (ODI).
Descriptive statistics are presented as frequencies and percentages for categorical variables. For continuous variables, means and 95% confidence intervals (CI) are presented unless non-normally distributed, in which case medians and inter-quartile ranges (IQR) are presented (e.g., EBL). Data distributions were examined visually through histograms and quantile-quantile plots. Differences between XLIF and ALIF were assessed with chi-squared and Fisher’s exact tests for categorical variables, independent samples t-tests for normally distributed continuous variables, and Mann Whitney U tests for non-normally distributed continuous variables. Pre- to postoperative differences in radiographic parameters were assessed with paired t-tests. Multivariable generalized estimating equations (GEEs) were used to assess differences in OR time and EBL after adjusting for differences in relevant surgical details that differed between groups in univariate analyses. All analyses were performed with SAS version 9.4 (Cary, NC, USA) with a two-sided level of significance of α=0.05.
Results
Baseline factors
A total of 76 patients meeting the inclusion and exclusion criteria were identified: 55 patients undergoing ALIF and 21 patients undergoing XLIF at the single L4–5 level. There were no significant differences in patient age, sex, body mass index, presence of Charlson co-morbidities, presence of osteoporosis, or smoking status (Table 1). All patients had either grade 1 or 2 spondylolisthesis, although ALIF patients were more likely to have a grade 2 spondylolisthesis than XLIF patients (62.2% vs. 26.3%, P=0.023). There were no significant difference in the proportion of patients with stenosis or previous spine surgery between groups (Table 1).
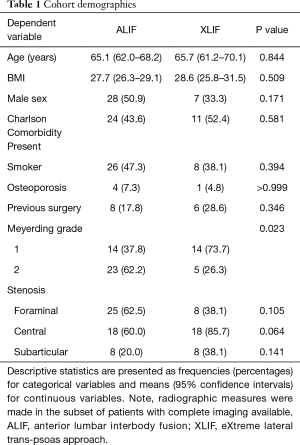
Full table
Surgical details
There were no standalone cases in either group, and no significant difference in rate of use of pedicle screws or BMP use (Table 2). One XLIF case underwent interspinous fixation instead of pedicle screws. The implant material differed between groups (P<0.001), with structural femoral ring allograft used in 77.8%, and PEEK interbody graft used in 21.8% of ALIF patients, whereas PEEK interbody graft was used in all XLIF patients. Of note, there was a higher rate of posterior decompression during ALIFs than XLIFs (78.2% vs. 52.4%, P=0.034).
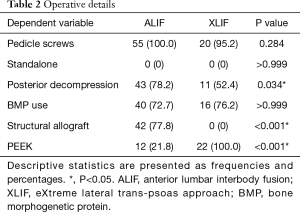
Full table
Perioperative outcomes
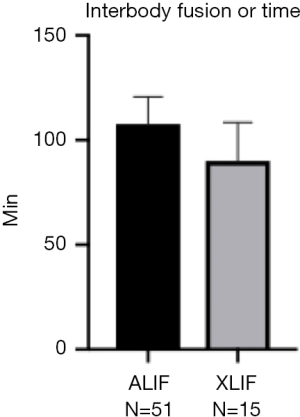
The median length of stay was 4 days for both groups (Figure 1). However, OR time was on average 40 min shorter for XLIF than for ALIF (P=0.026, Table 2, Figure 1). Interbody fusion time was available for a subset of patients (ALIF: n=51, XLIF: n=15), but did not differ between groups [ALIF 107 (IQR, 95–121) min, XLIF 91 (IQR, 72–108) min, P=0.174; Figure S1). In addition, the median estimated blood loss was 100 (IQR, 50–100) mL for XLIF, which was lower than that for ALIF (median 250; IQR, 150–400 mL; P<0.001, Figure 1). The OR time and EBL results were unchanged when the single case of interspinous suture fixation during an XLIF was excluded.

Multivariable analyses were undertaken to determine whether the differences in OR time and EBL persisted after accounting for the difference in posterior decompressions between groups. Posterior decompression was associated with an increase of 41.9 (95% CI: 8.4–75.3) min in OR time (P=0.015). After adjustment for this, the reduction in OR time with XLIF vs. ALIF was no longer significant but still trended towards being lower [difference: 31 (95% CI: −65.7 to 2.04) min, P=0.065]. Posterior decompression was not associated with EBL (P=0.842), and XLIF was still associated with lower EBL than ALIF after posterior decompression was included in the multivariable model [difference: 275 (95% CI: −520 to 30), P=0.028].
Postoperative complications
When looking at postoperative complications, 21.8% of ALIF patients and 38.1% of XLIF patients had a complication within 30 days (P=0.150, Table 3). With the available sample size, there were no statistically significant differences in complication rates, both when considered either together or individually. Beyond 1 month, 5 patients undergoing ALIF (9.3%) underwent re-operation at the L4–5 level, compared to none in the XLIF group, though this result was also not statistically significant. Re-operations in the ALIF group were revision decompressions at the same level. Post-operative leg pain was higher in the ALIF group than the XLIF group, but this was not significant. There was no difference in rates of transient paresthesias between groups. Other complications reported were urinary catheter reinsertions for retention, urinary tract infections, and post-operative atrial fibrillation, for which there were no significant differences between groups. There were no re-operations for failed fusions.

Full table
Radiographic parameters
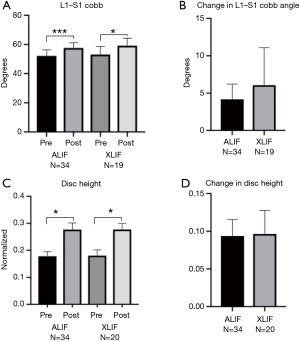
Radiographs were available for a subset of patients (n=45 ALIF, n=19 XLIF). Median radiographic follow-up was comparable in the ALIF group (median 13.27 months, minimum 3.23 months, maximum 105.00 months) and XLIF group (median 20.7 months, minimum 6.17 months, maximum 100.00 months; Table 4). Both ALIF and XLIF resulted in increased L1–S1 Cobb angles increases post-operatively compared to pre-operatively (P<0.001 for ALIF, P=0.021 for XLIF, Figure 2). However, the magnitude of the increase did not differ between groups (P=0.396). Both ALIF and XLIF increased L4–5 disc height (ALIF P<0.001, XLIF P<0.001), with the increase again not differing between groups (P=0.879). All cases demonstrated solid bony fusion, although XLIF group had a higher rate of Grade 1 fusions than ALIF (73.7% vs. 37.8%; P=0.023; Table 4). There was no difference in rate of subsidence between groups and no revisions for pseudo-arthrosis.
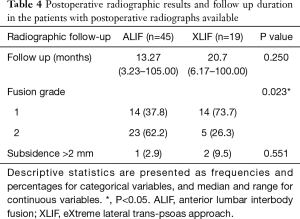
Full table
Patient reported outcomes
Patient reported outcomes were available for only a small subset of patients (ALIF n=13; XLIF n=9). The average follow-up time for these assessments was roughly 4.5 (95% CI: 4.0–4.9) years for the NRS and 4.4 (95% CI: 3.9–4.9) years for the ODI. NRS (ALIF n=13 back, n=12 leg; XLIF n=5 back, n=7 back) and ODI (ALIF n=12, XLIF n=9) measures were available for a limited subset of patients from the ALIF and XLIF groups (Figure 3). In this subset of patients, both ALIF and XLIF groups demonstrated pre- to postoperative improvement in NRS leg and back pain scores and ODI scores (P<0.001 for each, Figure 3). Median improvement in NRS leg pain was 4.2 (95% CI: 1.5–6.8) in ALIF and 6.0 (95% CI: 0.0–8.0) in XLIF. Median improvement in NRS back pain was 2.3 (95% CI: 0.0–6.3) in ALIF and 6.0 (95% CI: 3.0–7.0) in XLIF. There were no significant differences in the pre- to post-operative changes in NRS leg (P=0.610) or back (P=0.553) pain or the ODI (P=0.915) between the ALIF and XLIF groups for those patients who completed both pre- and post-operative patient reported outcomes (Figure 3).

Discussion
Our results demonstrate that the XLIF approach for L4–5 degenerative spondylolisthesis was associated with decreased blood loss compared to the ALIF approach. Several studies have looked at complications or outcomes following lumbar interbody fusion from the two approaches, and others have compared interbody fusions at different levels, (e.g., ALIF L5–S1 vs. XLIF L4–5) (18,21,26-28). In this retrospective review we report our experience with XLIF vs. ALIF at L4–5 for degenerative spondylolisthesis.
One goal of the limited soft tissue dissection in XLIF approach is earlier patient mobilization. The lateral approach is characterized by a smaller incision, indirect decompression of neural elements, and unlike the anterior approach, does not require mobilization of vascular structures with ligation or sacrifice of the middle sacral or iliolumbar veins. Our results show significantly less blood loss from the lateral approach, even after adjusting for posterior decompression, which was performed more frequently in the ALIF group. Other studies have demonstrated less EBL in XLIF compared to posterior approaches at L4–5 for degenerative spondylolisthesis (29). A recent retrospective comparison of XLIF at L4–5 to ALIF at L5–S1 demonstrated less EBL (61 mL) with XLIF compared to ALIF (100 mL), although the difference was not significant (27). Another previous retrospective study demonstrated greater EBL with two level fusions compared to single level fusions (30). The same study demonstrated longer operative time in two-level fusions compared to single level fusions for XLIF. In the present study, the mean surgical time of 241 min in the XLIF group was greater than previous reports of 73–199 min (27,31-33), although removing the posterior decompression and percutaneous screw fixation resulted in a more comparable interbody fusion time 90.1 min (95% CI: 71.7–101.4). Furthermore, our median LOS of four days for the XLIF group was higher than the mean of 1.2–2.2 days previously described (27,31,34). Compared to previous studies, we note that our patients had significant comorbidities typical of an academic tertiary referral center.
Previous studies have demonstrated that for low grade spondylolisthesis, XLIF indirectly decompresses neural elements, and can mitigate the need for posterior decompression (20,34). In this study a lower rate of posterior decompressions occurred in the XLIF group. While the need for a posterior decompression presents one relative indication for a transforaminal approach, transforaminal approaches can also be limited by inadequate decompression of the contralateral nerve root and incomplete disc removal (35-37). For low grade spondylolisthesis, one can reliably perform indirect decompressions with the XLIF approach given the surface area and apophyseal ring contact for disc height restoration. While the ALIF approach provides indirect foraminal decompression as well (38), there may be need for further decompression. The presence of a posterior decompression can account at least in part for the increased operative time associated with ALIF in this study, although the increase in EBL in ALIF was independent of the additional posterior decompression,
Consistent with previous findings, there was no difference in rate of complications within thirty days between ALIF and XLIF, although our sample size limits this analysis (21,26). We observed a rate of 14% approach-related lower extremity paresthesias or pain after XLIF, in line with previous studies of 10–31% (26,28,29,32,34,39). Paresthesias or radicular symptoms have been previously reported in the ALIF population as well, at low rates (18). Previous studies have demonstrated low re-operation rates in XLIF (28,31,32,34), which we demonstrate is durable over longer mean follow-up.
Durable relief of leg and back symptoms was evident in the small subset of our patients with patient reported outcomes available. In this small subset, both XLIF and ALIF patients had improvements in both back and leg scores and the ODI, though these improvements did not differ between groups. Previous studies have estimated the minimum clinically important difference (MCID) in lumbar surgery as approximately 1.2 points on the scale for back, 1.6 points for leg, and approximately 13 points for the ODI (40). Improvements in both posterior and XLIF patient reported outcomes (29) as well as after ALIF (41) met these MCID.
Our study did have limitations. There were relatively few numbers of patients in each group which led to a lack of power to demonstrate significant differences between the approaches, particularly in detecting differences in complications and reoperation rates. While there were more posterior decompressions in the ALIF group, ALIF was still independently associated with increased EBL and a trend towards increased OR time. Small sample size limited power to detect significant differences in multivariate analysis as well as in interbody fusion time. The XLIF group also represented an early series for the surgeons performing the procedure and may have been influenced by a learning curve. A follow up study could compare complication rates with a more recent series. Our focus on a specific indication at a single level in order to more accurately compare the approaches resulted in a smaller sample size and a slightly narrower clinical application. Future studies will address larger patient populations.
Lumbar interbody fusion with decompression is an effective treatment for persistently symptomatic degenerative spondylolisthesis at L4–5. The XLIF approach is associated with diminished blood loss, without a noticeable increase in complications or compromise in patient outcomes.
Acknowledgments
None.
Footnote
Conflicts of Interest: I Cheng disclose the following conflicts of interest: AAOS: Board or committee member; Cervical Spine Research Society: Board or committee member; Empirical Spine: Research support; Globus Medical: IP royalties; Paid consultant; Nuvasive: IP royalties; Stock or stock Options; Scoliosis Research Society: Board or committee member; Spinal Cyte: Stock or stock Options; Spine Innovations: Stock or stock Options; Spine Wave: IP royalties; Stock or stock Options; SpineCraft: Paid consultant; Stryker: Paid consultant. The other authors have no conflicts of interest to declare.
Ethical Statement: This retrospective, single-institution study was approved by the Institutional Review Board at our academic institution (IRB #7935), and consent was waived by the IRB for the retrospective chart review.
References
- Hu SS, Tribus CB, Diab M, et al. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am 2008;90:656-71. [PubMed]
- Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am 2009;91:1295-304. [Crossref] [PubMed]
- Martin CR, Gruszczynski AT, Braunsfurth HA, et al. The surgical management of degenerative lumbar spondylolisthesis: a systematic review. Spine 2007;32:1791-8. [Crossref] [PubMed]
- Fischgrund JS, Mackay M, Herkowitz HN, et al. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine (Phila Pa 1976) 1997;22:2807-12. [Crossref] [PubMed]
- Rao PJ, Phan K, Giang G, et al. Subsidence following anterior lumbar interbody fusion (ALIF): a prospective study. J Spine Surg 2017;3:168-75. [Crossref] [PubMed]
- Ghogawala Z, Dziura J, Butler WE, et al. Laminectomy plus Fusion versus Laminectomy Alone for Lumbar Spondylolisthesis. N Engl J Med 2016;374:1424-34. [Crossref] [PubMed]
- Epstein NE. Commentary on: Laminectomy plus fusion versus laminectomy alone for lumbar spondylolisthesis by Ghogawala Z, Dziura J, Butler WE, Dai F, Terrin N, Magge SN, et al. NEJM 2016;374 (15):1424-34. Surg Neurol Int 2016;7:S644-7. [Crossref] [PubMed]
- Försth P, Ólafsson G, Carlsson T, et al. A Randomized, Controlled Trial of Fusion Surgery for Lumbar Spinal Stenosis. N Engl J Med 2016;374:1413-23. [Crossref] [PubMed]
- Rampersaud YR, Fisher C, Yee A, et al. Health-related quality of life following decompression compared to decompression and fusion for degenerative lumbar spondylolisthesis: a Canadian multicentre study. Can J Surg 2014;57:E126-33. [Crossref] [PubMed]
- Kelleher MO, Timlin M, Persaud O, et al. Success and Failure of Minimally Invasive Decompression for Focal Lumbar Spinal Stenosis in Patients With and Without Deformity. Spine (Phila Pa 1976) 2010;35:E981-7. [Crossref] [PubMed]
- Chang HS, Fujisawa N, Tsuchiya T, et al. Degenerative spondylolisthesis does not affect the outcome of unilateral laminotomy with bilateral decompression in patients with lumbar stenosis. Spine (Phila Pa 1976) 2014;39:400-8. [Crossref] [PubMed]
- Wiltse LL, Bateman JG, Hutchinson RH, et al. The paraspinal sacrospinalis-splitting approach to the lumbar spine. J Bone Joint Surg Am 1968;50:919-26. [Crossref] [PubMed]
- Le VH, Lebwohl NH. Spondylolisthesis: A Historical Perspective on Etiology, Diagnosis, and Treatment. In: Wollowick AL, Sarwahi V. editors. Spondylolisthesis: Diagnosis, Non-Surgical Management, and Surgical Techniques. Boston, MA: Springer US; 2015:3-15.
- Resnick DK, Watters WC, Sharan A, et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 9: lumbar fusion for stenosis with spondylolisthesis. J Neurosurg Spine 2014;21:54-61. [Crossref] [PubMed]
- Ozgur BM, Aryan HE, Pimenta L, et al. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 2006;6:435-43. [Crossref] [PubMed]
- Khajavi K, Shen A, Lagina M, et al. Comparison of clinical outcomes following minimally invasive lateral interbody fusion stratified by preoperative diagnosis. Eur Spine J 2015;24 Suppl 3:322-30. [Crossref] [PubMed]
- Youssef JA, McAfee PC, Patty CA, et al. Minimally invasive surgery: lateral approach interbody fusion: results and review. Spine (Phila Pa 1976) 2010;35:S302-11. [Crossref] [PubMed]
- Malham GM, Parker RM, Blecher CM, et al. Choice of Approach Does Not Affect Clinical and Radiologic Outcomes: A Comparative Cohort of Patients Having Anterior Lumbar Interbody Fusion and Patients Having Lateral Lumbar Interbody Fusion at 24 Months. Global Spine J 2016;6:472-81. [Crossref] [PubMed]
- Takahashi K, Kitahara H, Yamagata M, et al. Long-term results of anterior interbody fusion for treatment of degenerative spondylolisthesis. Spine (Phila Pa 1976) 1990;15:1211-5. [Crossref] [PubMed]
- Marchi L, Abdala N, Oliveira L, et al. Stand-alone lateral interbody fusion for the treatment of low-grade degenerative spondylolisthesis. ScientificWorldJournal 2012;2012:456346. [Crossref] [PubMed]
- Winder MJ, Gambhir S. Comparison of ALIF vs. XLIF for L4/5 interbody fusion: pros, cons, and literature review. J Spine Surg 2016;2:2-8. [Crossref] [PubMed]
- Bridwell KH, Lenke LG, McEnery KW, et al. Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine (Phila Pa 1976) 1995;20:1410-8. [Crossref] [PubMed]
- Choi JY, Sung KH. Subsidence after anterior lumbar interbody fusion using paired stand-alone rectangular cages. Eur Spine J 2006;15:16-22. [Crossref] [PubMed]
- Cheung KM, Zhang Y, Lu D, et al. Reduction of Disc Space Distraction After Anterior Lumbar Interbody Fusion With Autologous Iliac Crest Graft. Spine 2003;28:1385-9. [Crossref] [PubMed]
- Meyerding HW. Spondylolisthesis; surgical fusion of lumbosacral portion of spinal column and interarticular facets; use of autogenous bone grafts for relief of disabling backache. J Int Coll Surg 1956;26:566-91. [PubMed]
- Hrabalek L, Adamus M, Gryga A, et al. A comparison of complication rate between anterior and lateral approaches to the lumbar spine. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2014;158:127-32. [Crossref] [PubMed]
- Xu DS, Bach K, Uribe JS. Minimally invasive anterior and lateral transpsoas approaches for closed reduction of grade II spondylolisthesis: initial clinical and radiographic experience. Neurosurg Focus 2018;44:E4. [Crossref] [PubMed]
- Ozgur BM, Agarwal V, Nail E, et al. Two-year clinical and radiographic success of minimally invasive lateral transpsoas approach for the treatment of degenerative lumbar conditions. SAS J 2010;4:41-6. [Crossref] [PubMed]
- Sembrano JN, Tohmeh A, Isaacs R, et al. Two-year Comparative Outcomes of MIS Lateral and MIS Transforaminal Interbody Fusion in the Treatment of Degenerative Spondylolisthesis: Part I: Clinical Findings. Spine (Phila Pa 1976) 2016;41 Suppl 8:S123-32. [PubMed]
- Knight RQ, Schwaegler P, Hanscom D, et al. Direct lateral lumbar interbody fusion for degenerative conditions: early complication profile. J Spinal Disord Tech 2009;22:34-7. [Crossref] [PubMed]
- Rodgers WB, Gerber EJ, Patterson J. Intraoperative and Early Postoperative Complications in Extreme Lateral Interbody Fusion: An Analysis of 600 Cases. Spine (Phila Pa 1976) 2011;36:26-32. [Crossref] [PubMed]
- Rodgers JA, Gerber EJ, Lehmen JA, et al. Clinical and Radiographic Outcome in Less Invasive Lumbar Fusion: XLIF at Two Year Follow-Up. J Spine Neurosurg 2013;2:3. [Crossref]
- Campbell PG, Nunley PD, Cavanaugh D, et al. Short-term outcomes of lateral lumbar interbody fusion without decompression for the treatment of symptomatic degenerative spondylolisthesis at L4-5. Neurosurg. Focus 2018;44:E6. [Crossref] [PubMed]
- Oliveira L, Marchi L, Coutinho E, et al. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976) 2010;35:S331-337. [Crossref] [PubMed]
- Phan K, Thayaparan GK, Mobbs RJ. Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion - systematic review and meta-analysis. Br J Neurosurg 2015;29:705-11. [Crossref] [PubMed]
- Hsieh PC, Koski TR, O’Shaughnessy BA, et al. Anterior lumbar interbody fusion in comparison with transforaminal lumbar interbody fusion: implications for the restoration of foraminal height, local disc angle, lumbar lordosis, and sagittal balance. J Neurosurg Spine 2007;7:379-86. [Crossref] [PubMed]
- Faundez AA, Schwender JD, Safriel Y, et al. Clinical and radiological outcome of anterior-posterior fusion versus transforaminal lumbar interbody fusion for symptomatic disc degeneration: a retrospective comparative study of 133 patients. Eur Spine J 2009;18:203-11. [Crossref] [PubMed]
- Rao PJ, Maharaj MM, Phan K, et al. Indirect foraminal decompression after anterior lumbar interbody fusion: a prospective radiographic study using a new pedicle-to-pedicle technique. Spine J 2015;15:817-24. [Crossref] [PubMed]
- Bendersky M, Solá C, Muntadas J, et al. Monitoring lumbar plexus integrity in extreme lateral transpsoas approaches to the lumbar spine: a new protocol with anatomical bases. Eur Spine J 2015;24:1051-7. [Crossref] [PubMed]
- Copay AG, Glassman SD, Subach BR, et al. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and Pain Scales. Spine J 2008;8:968-74. [Crossref] [PubMed]
- Flouzat-Lachaniette CH, Ratte L, Poignard A, et al. Minimally invasive anterior lumbar interbody fusion for adult degenerative scoliosis with 1 or 2 dislocated levels. J Neurosurg Spine 2015;23:739-46. [Crossref] [PubMed]


