Feasibility of local administration of chemotherapeutic drugs as an effective adjuvant therapy in primary, recurrent and metastatic extradural tumours of the spine—review
Background
Primary epidural spine tumours constitute 10% of the tumours that involve the bony spine (1). Spine is also a common site for metastasis seen in up to 40% of cancer patients. 10% to 20% of patients with spinal metastasis develop paralysis (2-4). Local recurrence has been estimated to occur in 10% to 20% of these patients (5-7) and is considered as the worst complication in the management of spinal tumours as it significantly affects the quality of life and prognosis. This highlights the importance of achieving good local control in the management of spine tumours (8). Present day multimodality treatment with advances in systemic chemotherapy and radiotherapy has increased the survival of patients significantly even in those primary tumours which were once considered to have a poor prognosis. However, local recurrence can severely jeopardise the quality of life and even reduce survival. Thus, it is imperative that we attain tumour control to the maximum extent possible during the index surgery to reduce the chance of recurrence. En bloc resections with negative margins have been shown to have the least recurrence following surgery for primary and metastatic spine tumours. Nevertheless, en bloc resections are always not possible in the management of spine tumours due to the anatomy and close vicinity to the cord and major blood vessels and are often intolerable in those patients with co-morbid conditions and limited life expectancy (6,9-11). Thus, in those instances where the excision was intralesional or with evidence of breach of tumour margin, a local strategy to cause destruction of residual tumour cells is highly desirable. Systemic chemotherapy is often not the first line management for spine tumours and metastasis because of the fear of side effects that would occur even before therapeutic levels could be reached in the bone (12,13). In such instances, local administration of chemotherapy might be an attractive option to ensure eradication of remaining tumour cells after excision. Any such attempt to reduce the chance of recurrence could have a significant positive impact on the functional and quality of life measures, even though it may not significantly affect the overall prognosis in patients as it is predominantly based on the extent of systemic spread (14). Local treatment has already been recognised as a treatment strategy in the management of bladder cancer with Bacillus Calmette-Guerin (BCG) (15), gliomas in the brain with intratumoral or intracavitary chemotherapy (16,17) and in hepatocellular carcinomas with percutaneous ethanol or chemoembolisation (18). Similar strategies are also being investigated in the other visceral cancers like the lung. Nevertheless, local administration of chemotherapy still largely remains as an unexplored treatment alternative with few available literatures already supporting its efficacy as an effective adjuvant therapy.
Objectives
The objectives of the present review are to look for evidence in the English literature on
- The efficacy of local administration of chemotherapeutic drugs in the management of primary and metastatic spine tumours;
- The suitability of chemotherapy drugs for local administration following surgical treatment of primary and metastatic spine tumours;
- The suitability of the delivery methods for local administration of chemotherapy;
- The possibility of failure mechanisms and adverse effects following this technique.
Methodology
Search strategy—a comprehensive review of the English literature was performed using PubMed, MEDLINE, EMBASE, SCOPUS, Google Scholar, Web of Knowledge, Biomed Central and Cochrane Database of Systematic reviews. Search terms included the MeSH terms/keywords: “perioperative", “local administration”, “intratumoral”, “intracavitary”, “interstitial”, “chemotherapy and related terms” and “spine tumours”.
When we began the literature search, we intended to conduct a proper systematic review and applied the inclusion and exclusion criteria as described in Table 1. We initially searched for studies only which used local administration of chemotherapeutic drugs in primary and metastatic spine tumours in humans. As only one case series study could be found, we relaxed our inclusion and exclusion criteria to include the animal studies and recurrent tumours as well and present our findings only as literature review. For the purpose of this review, only studies involving local administration of chemotherapeutic drugs for the management of spinal tumours were included. Data relevant to the research questions were recorded in tabular form.

Full table
Results
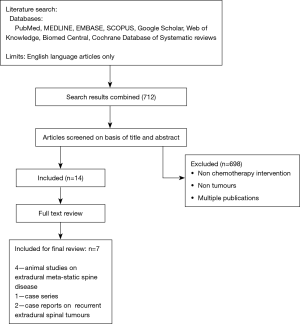
The initial search of PubMed, MEDLINE, EMBASE, SCOPUS, Google Scholar, Web of Knowledge, Science Direct, ProQuest, Sage, Biomed Central and Cochrane Database of Systematic reviews resulted in 712 references. After applying the inclusion and exclusion criteria (Table 1) and reviewing the full text articles, 4 animal studies on extradural metastatic spine disease, 1 human case series on spinal liposarcoma and 2 human case reports on recurrent extradural spinal tumours were found to be relevant (Figure 1).
Discussion
Skeletal tissue is one of the most frequent sites for metastasis of malignant tumours. Survival of metastasis patients is directly correlated with osseous involvement especially spinal cord compression (19-21). Perioperative local administration of chemotherapeutic drugs has many theoretical advantages. (I) It avoids the systemic side effects of systemic chemotherapy which includes myelosuppression, cardiotoxicity, renal toxicity, infection and gastrointestinal side effects. Previous studies have shown that high systemic levels of chemotherapy drugs do not reach an effective concentration in tumours (22). Therefore, there is a possibility that high systemic levels of a chemotherapy drug often lead to systemic toxicity without even achieving cytotoxic concentration locally at the tumour area; (II) it avoids the organ damage from chemotherapy drugs and reduces the incidence of chemotherapy induced secondary cancers especially in children; (III) it achieves a high concentration of chemotherapy drug within a tumour and increased exposure time for a tumour to the drug which cannot be achieved by systemic therapy, thereby maximising the clearance of tumour cells. As only 10% to 15% of tumour cells are believed to be in the active replication phase at any time, an increased exposure time of the tumour to the drug is important to kill the residual tumour cells which replicate at different points of time (23,24); (IV) it is especially useful in drugs which have a shorter half-life or gets cleared from the body sooner. For instance, Carmustine has a shorter half-life of 15 min. But by sustained delivery systems, tumoricidal concentration can be achieved for up to 21 days in animal models (25); (V) it can potentially avoid the need for postoperative chemotherapy and radiotherapy and may reduce the health care costs associated with chemotherapy and radiotherapy; (VI) some chemotherapeutic drugs like paclitaxel have radiosensitising properties which blocks tumour cells in the radiosensitive phases of cell cycle (G2M phase). This technique which ensures a high concentration of drug close to the operated site is most likely to increase the benefits of subsequent radiotherapy (26,27); (VII) single time application of chemotherapy drug and thereby resulting in less wastage of drug (24).
However, this technique is not without any limitations which include: (I) high concentration of the drug is seen close to the delivery device or the site of administration and those tumour cells away from the device may escape from the effect of the drug. This limitation can be mitigated to some extent through controlled drug-eluting device; (II) non-uniform distribution of drugs within the residual tumour mass or target region; (III) complications like wound healing problems, chemical and bacterial meningitis and subdural empyema have also been reported in animal (28) and human studies (29).
Rationale for using chemotherapy locally as an adjuvant therapy following excision
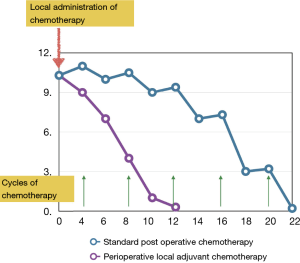
Human cancers follow Gompertzian model of growth as opposed to the log-kill kinetics which was once popular during the early years of the modern chemotherapy era. Log kill kinetics model was based on the belief that tumour growth is logarithmic, with the ratio of proliferating cells to total cells being constant. As a result, cell death is proportional regardless of tumour burden. However, this model became unpopular later as most human cancers were found to not follow purely exponential growth (30). Rather they follow the Gompertzian model of growth in which the ratio of proliferating cells to total cells reduces with increasing tumour size with a resultant exponential decline in growth fraction over time. Thus, in this model, chemotherapy which targets rapidly multiplying cells, kills an only small fraction of cells when the tumour is large and advanced but on the other hand, kills a larger fraction of cells when the tumour is small or clinically undetectable. This is especially important in the scenario of residual tumour cells which regrows following excision (31). The microscopic or macroscopic tumour deposits following excision have higher growth fraction resulting in faster regrowth of tumour. The residual tumour cells continue to multiply till the time they are attacked by the adjuvant therapy (chemotherapy or radiotherapy) which are usually given in cycles rather continuously to evade the systemic effects from them. Also, residual cells continue to multiply when they are not exposed to the chemotherapy drugs as in the time interval between the cycles. This limitation is likely to be surpassed when chemotherapeutic drugs are administered locally following surgical excision of tumours allowing no time for the tumour cells to multiply due to constant exposure to the drug leading to a steeper decline in the actual tumour burden.
Figure 2 details the theoretical possibility of residual tumour cell growth kinetics following surgical excision when exposed to the chemotherapy drugs locally.
Local administration of chemotherapeutic drugs—is it an effective adjuvant therapy for the management of spine tumours?
Table 2 lists the various studies where local administration of chemotherapeutic agents has been studied in relation to the management of spine tumours. At the time of writing this review, only 4 animal studies, 2 case reports and 1 case series were found in relation to the management of spine tumours despite repeated extensive literature search. Abe et al. in his 2007 and 2008 spine metastasis rat model study, demonstrated a statistically significant improvement in the disease-free time and survival when treated with paclitaxel-eluting drug delivery device (6,32). Bagley et al. also established a similar benefit with no systemic toxicity in breast carcinoma metastasis rat model when treating with a different drug delivery device eluting paclitaxel (33). Another study which implanted local administration of paclitaxel as adjuvant therapy in a breast carcinoma metastasis rat model showed added benefit in those rats where this technique was used in combination with surgery and radiotherapy than in those treated with surgery and radiotherapy alone (14).
Full table
The following study mentioned the use of intraoperative chemotherapy. Zhao et al. reported a case series of 7 patients with spinal liposarcoma who were managed surgically with resection and reconstruction. One en bloc resection and six piecemeal resections were performed. All patients had intraoperative chemotherapy with cisplatin with the aim to reduce residual tumour cells and thereby local recurrence. It was performed by rinsing and soaking the surgical field with cisplatin dissolved by desalted water. At the end of follow-up period (average =24.6±13.9 months), 2 patients had local recurrence requiring repeat operation, 3 patients were alive with no evidence of disease and 2 died because of disease and complications. However, there were few concerns in this study as well. Firstly, there was no standardisation of cisplatin administration following resection the study neither mentioned the dose nor there was sustained delivery of drug at the operated site. Secondly, only one application with short exposure time might not be very beneficial in eradicating the remaining tumour cells as they enter the most sensitive phase for the drug to act at different time periods. Importantly, the study did not mention any adverse effects secondary to one-time application of chemotherapy drug (34).
The possible benefits of this technique have also been shown in 2 human case reports with recurrent spinal tumours. Duncan et al. in his case report of recurrent angiosarcoma of C3, C4 treated previously with surgery, radiotherapy and chemotherapy noticed a good clinical improvement in terms of neurological recovery and regression of tumour size in MRI following intratumoral injection of Bleomycin. In addition, no systemic toxicity was observed due to bleomycin injection (35).
Another case report of recurrent chordoma C5, C6 treated previously by corpectomy also showed significant clinical improvement with regression of spinal cord compression and 42% reduction in the size of the tumour following intratumoral administration of carboplatin and epinephrine. No systemic toxicity to carboplatin or epinephrine was noticed (36).
Chemotherapy drugs suitable for local administration
The first report of using a chemotherapeutic agent for intratumoral delivery came from Heppner and Diemath in 1963 who used endoxan soaked spongastan (37). Since then, many chemotherapy medicines from different groups have been tested as a potential drug for local administration in a variety of tumours (24). These include vincristine (38), doxorubicin (39), bleomycin (40), cisplatin (36), carmustine (41), 5-fluorouracil (42), methotrexate (43) and paclitaxel. Among these, Paclitaxel has been shown to have significant antitumour activity in solid cancers including breast cancer. Being a taxane group drug, it has dose dependent anti-proliferative, anti-angiogenic, anti-metastatic and apoptotic activities. Local administration of paclitaxel exhibits well balanced therapeutic effects than when administered systemically. Additionally, its radiosensitizing property is an added advantage for better local tumour control as surgery followed by radiotherapy is the usual norm for the management of spine tumours rather than an exception. Paclitaxel being extremely hydrophobic, intravenous administration is limited and in addition, a formulation containing cremophor EL has been found to cause hypersensitivity reactions. All animal studies included in the present review had used paclitaxel as the chemotherapeutic agent for local administration. However, not all chemotherapeutic drugs are amenable to a local administration. For instance, cyclophosphamide which is the most widely used anticancer agent is a poor candidate for local administration as it requires enzymatic activation to an active metabolite in the liver by the p450 cytochrome oxidase system. The parent compound as such has no antitumour activity.
Delivery modes for local administration of cancer drugs
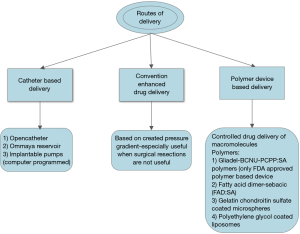
Figure 3 outlines the various drug delivery methods that have been tried for the local administration of chemotherapy. Polymer-based delivery is more popular among the other types due to the advantage of controlled drug delivery. In 1976, Langer and Folkman introduced the polymer-based drug delivery system using the polymer Ethylene Vinyl acetate copolymer (EVAc) (44). Since then, various polymer systems have evolved for delivery of a variety of drugs including steroids and anti-inflammatories. Till this date, Gliadel, a PCPP:SA {Poly[bis(p-carboxyphenoxy propane-sebacic acid)]} polymer loaded with carmustine is the only FDA approved device approved for the treatment of gliomas. This system allows moulding into various shapes like rods, wafers, sheets and microspheres due to the requirement for high pressures and low temperatures needed for the synthesis (45). Preclinical and clinical trials have ensured the safety of this polymer and no additional toxicity was seen when combined with radiotherapy postoperatively (46). This advantage can also be exploited in the treatment of spine tumours by customising the shape of the polymer into the possible shape of the defect that will result following the excision of a tumour. Future researches on this area can result in the synthesis of these polymers which can provide structural stability as well.
Similarly, hydroxyapatite alginate composites have also been found to produce promising results as one of the best carriers for the treatment of bone diseases. Hydroxyapatite possesses osteoconductive property even in irradiated bones and is considered better than other biomaterials. Alginate possesses cell adhesion quality and sustained release of drugs. Abe et al., in his 2007 murine study randomised ten rats into two groups—local treatment group and control group, and utilised paclitaxel loaded hydroxyapatite alginate gel and demonstrated that in the local treatment group, survival has increased by an average of 7.6 days in the local treatment group (21.6 days) from 14 days in the control group. Additionally, rats in local treatment group demonstrated better hindlimb motor function [using Basso-Beattie-Bresnahan (BBB) rating scale]. Another study by the same author in 2008 used paclitaxel loaded hydroxyapatite alginate beads. Twenty one rats were grouped into (I) control group; (II) local treatment group where paclitaxel loaded hydroxyapatite alginate beads were implanted intraosseously; (III) systemic group. It was found that local treatment group had 140% to 150% increase in survival and disease-free time. The same was not observed in the systemic group even after administration of 30 times higher dose (32).
Oncogel (Protherics, Inc., USA) is another polymer based drug delivery system consisting of paclitaxel-loaded in a water-soluble, biodegradable polymer (poly-DL-lactide-co-glycolide-polyethylene glycol-poly-DL-lactide-co-glycolide) with a physical property of transformation into a gel state from liquid state once the temperature exceeds 17.8 °C. It has the advantage of sustained release of paclitaxel from the gel in a linear fashion over approximately the next 50 days (47). The combination of Oncogel’s gelled state and sustained drug release from it makes it another attractive treatment option. Bagley et al. in 2007, used Oncogel loaded with paclitaxel in two different concentrations—3% and 6% and established that rats with Oncogel 3% and 6% had longer survival period (average of 18 days) than the control group which survived for 14 days (statistically significant P<0.05). In addition, more necrosis with less infiltration was seen in the group treated with 6% Oncogel than compared to other treatment groups. In addition, no signs of systemic toxicity were noted in any of the treatment groups (33). Gok et al., conducted an experimental study on rats using OncoGel as an adjuvant therapy in three different sets of experiments which include (I) OncoGel following surgery versus control group; (II) OncoGel following surgery and Radiotherapy versus control group and (III) Oncogel following Radiotherapy versus control group. In each experimental group, animals which received OncoGel fared best with respect to delay in loss of hindlimb function (BBB scale). He concluded that best treatment option for spinal metastasis would be the combination of surgery with radiation and OncoGel (14).
Cancer drugs loaded cement has also been explored as one of the potential tools for both local deliveries of therapeutic substances and structural support (48,49). polymethyl methacrylate (PMMA) polymer in bone cement has been used as a carrier vehicle for drugs like antibiotics (50) and nonsteroidal anti-inflammatory drugs (NSAIDs) (51) since the 1970s. Hernigou et al. for the first time in 1989 proved the release of methotrexate from methotrexate loaded cement implanted in patients with bone tumours (52). It has also been demonstrated that loading of cement with antiblastic compounds like doxorubicin, cisplatin, methotrexate by in vitro and in vivo studies did not affect the polymerisation of PMMA. The addition of the drug neither altered the cytotoxic effect nor the biomechanical properties of the cement. This study has also demonstrated the cytotoxic effect of the eluted drug on osteosarcoma cell lines.
In addition to the PMMA cement, calcium phosphate cement and aluminium free glass ionomer cement have also been studied for local administration of chemotherapy.
Safety
Researches in brain tumours with interstitial or intratumoral chemotherapy have proved the safety of this method on neural tissues. Intratumoral or interstitial chemotherapy was devised to bypass the barriers [blood brain barrier, blood cerebrospinal fluid (CSF) barrier and blood tumour barrier] and deliver a high concentration of drug to the tumour locally and avoid the systemic effects of chemotherapy (23). This process has not been found to cause additional toxicity to the neural tissues. This, when extrapolated to the treatment of primary tumours and metastatic spine tumours, would most likely to be safe for the spinal cord too. In addition to the above effective similar barriers in the spinal cord, dura being a tough structure can provide additional protection to the cord from the direct cytotoxic effects of chemotherapeutic drugs. Tyler et al., in his murine intramedullary gliosarcoma tumour model clearly demonstrated that OncoGel can be administered safely into the spinal cord of rats at doses unto 5 microsites of 3.0 mg/mL of paclitaxel with significant prolongation of hindlimb motor function and survival. However, a dose of 5 mL of 6.0 mg/mL caused rapid deterioration of hindlimb motor function which can be attributed to the spinal cord edema secondary to paclitaxel (27).
The limitations of this review are all studies mentioned here determine the effect of single dose application of treatment before onset of neurological deficits. This is true to some extent when applied to the clinical setting as 15% to 40% of patients with metastatic spine tumour disease do not have an obvious motor weakness at the time of diagnosis. All studies mentioned neurological decline as a measure of therapeutic outcome rather than imaging. Further, no information is available on the status of wound healing, tumour regression following the adjuvant local chemotherapy.
Another important limitation of animal study is that it did not take into account of other important issues like instability which is more obvious in the upright human than in the quadruped rat. The complex systemic and biomechanics factors seen in human primary and metastatic spine tumours cannot be replicated in an animal model and hence the results from the animal studies cannot be directly applied to the human setting. However, they provide an important preclinical data suggesting that local administration of chemotherapy following surgical resection is likely to produce improved functional outcomes than compared to the present treatment regimen of surgery and irradiation alone or surgery and irradiation in combination (14).
Some of the animal studies included in our review have used non- human cell lines for creating tumour models in rats. There can be a significant difference in the response of tumour cells to paclitaxel which needs to be taken into account as well.
The available data from all the experimental studies on animal models provide initial evidence that local chemotherapy following surgical excision +/– radiotherapy may have an additive effect and needs to be further explored in the form of clinical trials in humans.
Similar to the local administration of chemotherapeutic agents, various immunotherapeutic agents as potential targets for intratumoral or intralesional administration are currently under trial in both preclinical and clinical settings (53). Concurrently, advances in drug delivery systems like polymersomes and nanoparticle technology will soon revolutionise the way we treat primary and metastatic spinal tumours (54). A detailed description of the above technology is beyond the scope of this article.
Conclusions
The technique of local administration of chemotherapeutic agents as an adjuvant therapy following excision of primary and metastatic spine tumours would be a novel strategy to ensure a tumour free environment. This would prove to be very useful as it reduces the chances of recurrence, reduces morbidity and potentially improves quality of life and prognosis. Future clinical trials should be conducted to prove the efficacy of this useful adjuvant treatment modality.
Acknowledgments
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Sundaresan N, Rosen G, Boriani S. Primary malignant tumors of the spine. Orthop Clin North Am 2009;40:21-36. [Crossref] [PubMed]
- Böhm P, Huber J. The surgical treatment of bony metastases of the spine and limbs. J Bone Joint Surg Br 2002;84:521-9. [Crossref] [PubMed]
- Witham TF, Khavkin YA, Gallia GL, et al. Surgical insight: current management of epidural spinal cord compression from metastatic spine disease. Nat Clin Pract Neurol 2006;2:87-94. [Crossref] [PubMed]
- Klimo P Jr, Schmit MH. Surgical management of spinal metastases. Oncologist 2004;9:188-96. [Crossref] [PubMed]
- Oka H, Kondoh T, Seichi A, et al. Incidence and prognostic factors of Japanese breast cancer pa- tients with bone metastasis. J Orthop Sci 2006;11:13-9. [Crossref] [PubMed]
- Abe T, Sakane M, Ikoma T, et al. Intrasosseous delivery of paclitaxel loaded hydroxyapatite - alginate composite beads delaying paralysis caused by metastatic spine cancer in rats. J Neurosurg Spine 2008;9:502-10. [Crossref] [PubMed]
- Schiff D. Spinal cord compression. Neurol Clin 2003;21:67-86. [Crossref] [PubMed]
- Aaron AD. The management of cancer metastatic to bone. JAMA 1994;272:1206-9. [Crossref] [PubMed]
- Boriani S, Biagini R, De Iure F, et al. En bloc resections of bone tumors of the thoracolumbar spine. A preliminary report on 29 patients. Spine 1996;21:1927-31. [Crossref] [PubMed]
- Fourney DR, Abi-Said D, Rhines LD, et al. Simultaneous anterior-posterior approach to the thoracic and lumbar spine for the radical resection of tumors followed by reconstruction and stabilization. J Neurosurg 2001;94:232-44. [PubMed]
- Tomita K, Kawahara N, Baba H, et al. Total en bloc spondylectomy: a new surgical technique for primary malignant vertebral tumors. Spine 1997;22:324-33. [Crossref] [PubMed]
- Lake DE, Hudis CA. High-dose chemotherapy in breast cancer. Drugs 2004;64:1851-60. [Crossref] [PubMed]
- Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002;2:584-93. [Crossref] [PubMed]
- Gok B, McGirl M, Scuibba DM, et al. Adjjuvant treatment with locally delivered OncoGel delays the onset of paresis after surgical resection of experimental spinal metastasis. Neurosurgery 2009;65:193-9. [Crossref] [PubMed]
- Morales A, Eidinger D, Bruce AW. Intracavitary bacillus Calmette-Guerin in the treatment of superficial bladder tumor. J Urol 1976;116:180-3. [Crossref] [PubMed]
- Haroun RI, Brem H. Local delivery. Curr Opin Oncol 2000;12:187-93. [Crossref] [PubMed]
- Walter KA, Tamargo RJ, Olivi A, Burger PC, Brem H. Intratumoral chemotherapy. Neurosurgery 1995;37:1128-45. [Crossref] [PubMed]
- Venook AP. Regional strategies for managing hepatocellular carcinoma. Oncology 2000;14:347-54. [PubMed]
- Sabbatini P, Larson SM, Kremer A, et al. Prognostic significance of extent of disease in bone in patients with androgen-independent prostate cancer. J Clin Oncol 1999;17:948-57. [Crossref] [PubMed]
- Soloway MS, Hardeman SW, Hickey D, et al. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer 1988;61:195-202. [Crossref] [PubMed]
- Taube T, Elomaa I, Blomqvist C, et al. Histomorphometric evidence for osteoclast-mediated bone resorption in metastatic breast cancer. Bone 1994;15:161-6. [Crossref] [PubMed]
- Shikanov A, Shikanov S, Vaisman B, et al. Cisplatin tumor biodistribution and efficacy after intratumoral injection of a biodegradable extended release implant. Chemother Res Pract 2011;;2011:175054. [Crossref] [PubMed]
- Walter KA, Tamargo RJ, Olivi A, et al. Intratumoral chemotherapy. Neurosurgery 1995;37:1128-45. [Crossref] [PubMed]
- Wolinsky JB, Colson YL, Grinstaff MW. Local drug delivery strategies for cancer treatment: gels, nanoparticles, polymeric films, rods, and wafers. J Control Release 2012;159:14-26. [Crossref] [PubMed]
- Grossman SA, Reinhard C, Colvin OM, et al. The intracerebral distribution of BCNU delivered by surgically implanted biodegradable polymers. J Neurosurg 1992;76:640-7. [Crossref] [PubMed]
- Lapidus RG, Dang W, Rosen DM, et al. Anti-tumor effect of combination therapy with intratumoral controlled-release paclitaxel (PACLIMER microspheres) and radiation. Prostate 2004;58:291-8. [Crossref] [PubMed]
- Tyler BM, Hdieb A, Caplan J, et al. Delayed onset of paresis in rats with experimental intramedullary spinal cord gliosarcoma following intratumoral administration of the paclitaxel delivery system OncoGel. J Neurosurg Spine 2012;16:93-101. [PubMed]
- Pradilla G, Wang PP, Gabikian P, et al. Local intracerebral administration of Paclitaxel with the paclimer delivery system: toxicity study in a canine model. J Neurooncol 2006;76:131-8. [Crossref] [PubMed]
- Lidar Z, Mardor Y, Jonas T, et al. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J Neurosurg 2004;100:472-9. [Crossref] [PubMed]
- Norton L. Implications of kinetic heterogeneity in clinical oncology. Semin Oncol 1985;12:231-49. [PubMed]
- Hudis CA. Clinical implications of antiangiogenis therapies. Oncology 2005;19:26-31. [PubMed]
- Abe T, Sakae M, Tonegawa T, et al. Novel Local Treatment with Paclitaxel loaded Hydroxyapatite- alginate gels for spinal bone metastases. Key Engineering Materials 2007;330-332:1343-6. [Crossref]
- Bagley CA, Bookland MJ, Pindrik JA, et al. Local delivery of oncogel delays paresis in rat metastatic spinal tumor model. J Neurosurg Spine 2007;7:194-8. [Crossref] [PubMed]
- Zhao C, Han Z, Xiao H, et al. Surgical management of spinal liposarcoma: a case series of 7 patients and literature review. Eur Spine J 2016;25:4088-93. [Crossref] [PubMed]
- Duncan IC, Fourie PA, Alberts AS. Direct percutaneous intratumoral bleomycin injection for palliative treatment of impending quadriplegia. Am J Neuroradiol 2004;25:1121-3. [PubMed]
- Guiu S, Guiu B, Feutray S, et al. Direct Intratumoral chemotherapy with carboplatin and epinephrine in a recurrent cervical chordoma: case report. Neurosurgery 2009;65:E629-30. [Crossref] [PubMed]
- Heppner F, Diemath HE. Lokale chemotherapie der hirntumoren. Acta Neurochir (Wien) 1963;11:287-93. [Crossref] [PubMed]
- Oliver AS, Firth G, McKeran RO. Studies on the intracerebral injection of vincristine free and entrapped within liposomes in the rat. J Neurol Sci 1985;68:25-30. [Crossref] [PubMed]
- Lin SY, Cheng LF, Lui WY, et al. Tumoricidal effect of controlled-release polymeric needle devices containing Adriamycin HCl in tumor-bearing mice. Biomater Artif Cells Artif Organs 1989;17:189-203. [Crossref] [PubMed]
- Takahashi H, Nakazawa S, Shimura T. Evaluation of postoperative intratumoral injection of bleomycin for craniopharyngioma in children. J Neurosurg 1985;62:120-7. [Crossref] [PubMed]
- Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol 2003;5:79-88. [Crossref] [PubMed]
- Oda Y, Tokuriki Y, Tsuda E, et al. Trial of anticancer pellet in malignant brain tumours: 5 FU and urokinase embedded in silastic. Acta Neurochir Suppl (Wien) 1979;28:489-90. [PubMed]
- Nierenberg D, Harbaugh R, Maurer LH, et al. Continuous intratumoral infusion of methotrexate for recurrent glioblastoma: A pilot study. Neurosurgery 1991;28:752-61. [Crossref] [PubMed]
- Langer R, Folkman J. Polymers for the sustained release of proteins and other macromolecules. Nature 1976;263:797-800. [Crossref] [PubMed]
- Chasin M, Domb A, Ron E, et al. Polyanhydrides as drug delivery systems. In: Chasin M, Langer R. editors.Biodegradable polymers as drug delivery systems. NY: Marcel Dekker, 1990:43-70.
- Brem H, Tamargo RJ, Olivi A, et al. Biodegradable polymers for controlled delivery of chemotherapy with and without radiation therapy in the monkey brain. J Neurosurg 1994;80:283-90. [Crossref] [PubMed]
- Zentner GM, Rathi R, Shih C, et al. Biodegradable block copolymers for delivery of proteins and water-insoluble drugs. J Control Release 2001;72:203-15. [Crossref] [PubMed]
- Healey JH, Shannon F, Boland P, et al. PMMA to stabilize bone and deliver antineoplastic and antiresorptive agents. Clin Orthop Relat Res 2003.S263-75. [Crossref] [PubMed]
- Kiri L, Filiaggi M, Boyd D. Methotrexate-loaded glass ionomer cements for drug release in the skeleton: An examination of composition-property relationships. J Biomater Appl 2016;30:732-9. [Crossref] [PubMed]
- Bettencourt A, Almeida AJ. Poly (methyl methacrylate) particulate carriers in drug delivery. J Microencapsul 2012;29:353-67. [Crossref] [PubMed]
- Corry D, Moran J. Assessment of acrylic bone cement as a local delivery vehicle for the application of non-steroidal antiinflammatory drugs. Biomaterials 1998;19:1295-301. [Crossref] [PubMed]
- Hernigou P, Thie’ry JP, Benoit J, et al. Methotrexate diffusion from acrylic cement. Local chemotherapy for bone tumours. J Bone Joint Surg Br 1989;71:804-11. [Crossref] [PubMed]
- Ager C, Reilley M, Nicholas C, et al. 31st Annual Meeting and Associated Programs of the Society for Immunotherapy of Cancer (SITC 2016): part two. J Immunother Cance 2016;4:73. [Crossref]
- Lee JS, Feijen J. Polymersomes for drug delivery: Design, formation and characterization. Journal of controlled release 2012;161:473-83. [Crossref] [PubMed]