
Chemonucleolysis with chondroitin sulfate ABC endolyase as a novel minimally invasive treatment for patients with lumbar intervertebral disc herniation
Introduction
The use of chemonucleolysis as a minimally invasive treatment for cervical and lumbar intervertebral disc herniation (IDH) has been anticipated for more than 50 years (1). Although several enzymes (trypsin, hyaluronidase, cathepsin G, chymotrypsin, and calpain) have been proposed as therapeutic agents (2-6), only two enzymes (chymopapain and collagenase) have been used in clinical settings (1,7). Among them, chymopapain was introduced in July 1963 and became widely used throughout Europe, North America, and Australia (8-10). After reports of severe adverse events, including fatal anaphylaxis, bleeding, and neurologic complications (11-16), the production of chymopapain (Chymodiactin, Smith Laboratories Inc., MN, USA) for non-scientific and commercial reasons ceased in 2002. The first clinical study on collagenase (Nucleolysin) was reported in 1981 (7). Subsequently, collagenase was reported as being less injurious to spinal nerve roots and perineural tissues, but was reported as less clinically effective than chymopapain (17). Severe adverse events were also associated with this enzyme (cauda equina syndrome and disc herniation due to “digestion” of the annulus fibrosus) (8,18,19).
A safer and more effective chemonucleolysis enzyme has been expected, and as chondroitin sulfate ABC endolyase has shown promising results, it is finally ready to be launched in the Japanese market.
Chondroitin sulfate ABC endolyase
Chondroitin sulfate ABC endolyase is an enzyme that catalyzes the depolymerization of chondroitin sulfate. This enzyme cleaves the β-1,4-galactosaminic bonds between N-acetylgalactosamine and either D-glucuronic acid or L-iduronic acid. Consequently, chondroitin sulfate ABC endolyase degrades chondroitin A sulfate, chondroitin B sulfate, chondroitin C sulfate, and hyaluronic acid chains (Figure 1). Chondroitin sulfate is widely distributed in several mammalian tissues, including the nucleus pulposus (NP), skin, cornea, bone, and cartilage. Therefore, chondroitin sulfate ABC endolyase is a potential therapeutic enzyme for IDH. This enzyme produced by various bacterial strains has already been investigated. Among them, chondroitin sulfate ABC endolyase from Proteus vulgaris has been the most extensively examined for clinical application.
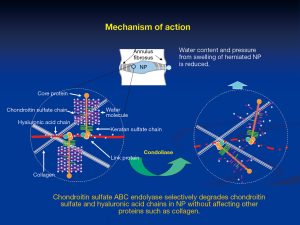
Proteus vulgaris chondroitin sulfate ABC endolyase was first purified by Yamagata et al. in 1968 (20). The characteristic activity was limited to the abovementioned side chains, but did not affect keratan sulfate, heparin, or heparan sulfate. This activity was more specific than that of other enzymes (chymopapain and collagenase), which were previously used for chemonucleolysis. The gene of chondroitin sulfate ABC endolyase was cloned by Sato et al. in 1994, and the active enzyme was expressed in Escherichia coli (21). Hamai et al. performed further purification of Proteus vulgaris chondroitin sulfate ABC endolyase from the fraction of exolyase activity (13).
Experimental studies have been performed using animal models such as hamsters, rabbits, pigs, dogs, and monkeys (12,22-27). All studies verified that chondroitin sulfate ABC endolyase was markedly less harmful than chymopapain to the surrounding tissues, nervous system, and vascular structures. Even with its selective and mild enzymatic effect, distinct clinical responses have been obtained in all animal models. For example, Takahashi et al. reported favorable outcome with chondroitin sulfate ABC endolyase in 59 dogs with symptoms and signs of cervical, thoracic, and lumbar IDH (12). Seven days after the injection of this enzyme, 45 of 48 dogs showed improved symptoms and signs. Not only no adverse reactions observed but also no recurrence was observed in nine dogs that were followed from 14 to 36 months after the injection.
Clinical applications
Considering these results, phases 1–3 clinical trials were performed in Japan. Matsuyama et al. reported results of phases 2 and 3 trials on chemonucleolysis with chondroitin sulfate ABC endolyase for the treatment of lumbar IDH (Clinical trial registration no.: NCT00634946) (11). In this study, a total of 194 patients received three different doses of chondroitin sulfate ABC endolyase (1.25, 2.5, or 5 U) or placebo injections, and pain intensity was measured using a 100 mm visual analog scale (VAS) (0 mm indicating “no pain” and 100 mm indicating “worst pain ever experienced”). Mean changes in the worst leg pain from baseline to week 13 was −31.7 mm (placebo), −46.7 mm (1.25 U), −41.1 mm (2.5 U), and −47.6 mm (5 U). The differences were significant at week 13 in the 1.25 U (P=0.03) and 5 U groups (P=0.01) compared with that in the placebo group. No patient died or developed anaphylaxis or neurological sequelae. The dose-response improvement in the worst leg pain at week 13 was not significant (P=0.14). The authors thus proposed the recommended clinical dose of chondroitin sulfate ABC endolyase as 1.25 U. Chiba et al. reported the results of phase 3 trials on chemonucleolysis with chondroitin sulfate ABC endolyase for lumbar IDH treatment (28). In this study, 163 patients received chondroitin sulfate ABC endolyase (1.25 U) or placebo injections, and the pain intensity was measured using a 100 mm VAS. Mean changes in the worst leg pain from baseline to week 52 was −42.3 mm (placebo) and −54.2 mm (chondroitin sulfate ABC endolyase). The difference was significant at week 52 in the chondroitin sulfate ABC endolyase group (P=0.01) compared with that in the placebo group. In the chondroitin sulfate ABC endolyase group, back pain, Modic type 1 change, and decrease in disc height were frequently reported without any clinically relevant consequence.
In Japan, chondroitin sulfate ABC endolyase (HERNICORE®, Seikagaku Corporation, Tokyo, Japan) was released in August 2018 for the treatment of lumbar IDH. To avoid unexpected adverse events, the first release was provided only to hospitals with abundant experience in the treatment of lumbar IDH. Additionally, the indication was limited to contained IDHs (subligamentous extrusion), excluding protrusion. Non-contained IDHs (transligamentous extrusion and sequestration) were excluded from the indication because of the risk of enzyme leakage and the low therapeutic effect in sequestrated NP fragment. Currently, chemonucleolysis with chondroitin sulfate ABC endolyase is being used only for patients with radiculopathy resistant to conventional conservative treatments.
Injection procedures
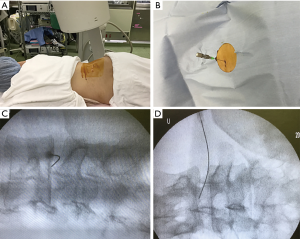
Patients were carefully log rolled into prone position. During the procedure, a fluoroscope was placed across the center of the operating table to ensure appropriate needle positioning (Figure 2A). In case of restricted fluoroscope movement, pelvic tilting was frequently used to confirm disc space and needle trajectory (similar to lateral recumbent position). After marking the skin entry position, the skin was disinfected and covered with a surgical drape. Under local anesthesia, 22-gauge spinal needle with stylet was inserted into the center of the disc space using a posterolateral approach from the opposite side of the radiculopathy (Figure 2B). The position of the needle tip was confirmed with a posterolateral fluoroscopic view during the insertion. The final tip position (the center of NP) was confirmed by both an anteroposterior and a lateral fluoroscopic view. The use of contrast medium was prohibited in the whole process. The stylet was pulled out, and 1.25 U of lyophilized chondroitin sulfate ABC endolyase dissolved in 1.2 mL of saline was injected. To respond to unexpected adverse events such as anaphylaxis, an intravenous line was established prior to injection, and an antibiotic was administered 30 min before the abovementioned procedures. The patients were hospitalized on the injection day.
Representative case
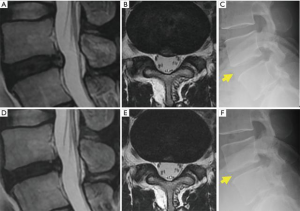
A representative case treated with chemonucleolysis using chondroitin sulfate ABC endolyase is shown in Figure 3. This 33-year-old man presented with left leg pain (L5 dermatome) that had started 3 months prior to visiting our outpatient clinic. Neurological examination revealed no apparent muscle weakness. Lumbar T2-weighted magnetic resonance imaging (MRI) revealed a central-type L4/5 lumbar IDH (Figure 3A). Leg pain relief after chondroitin sulfate ABC endolyase injection gradually occurred over a 2-week period and was associated with newly developed back pain. MRI performed 2 months after the injection revealed reduction of the lumbar IDH (Figure 3B) as well as reduction of the disc height. Brandner et al.’s disc index calculated from preoperative and postoperative radiographs revealed that the anterior Brandner et al.’s disc index had decreased from 0.375 to 0.195 (29). This change had been previously reported general course for the chemonucleolysis. Clinically, the patient complained of a newly developed persistent lumbago 3 months after the injection. This type of lumbago had already been reported in previous phase studies (11,28). As the pain could be controlled by medication, the patient was observed on an outpatient basis.
Discussion
Theoretically, chemonucleolysis seems to be the best possible strategy for the treatment for cervical and lumbar IDH. Although two enzymes (chymopapain and collagenase) had been previously used in clinical settings (1,7), severe adverse events such as anaphylaxis, bleeding, and neurologic complications were reported (11-16). Therefore, Proteus vulgaris chondroitin sulfate ABC endolyase was extensively analyzed early in the experimental studies for its effect on neural tissues, blood vessels, and other surrounding structures (e.g., yellow ligament and endplate) (12,22-27). All studies revealed that chondroitin sulfate ABC endolyase caused considerably less damage than chymopapain, except in NP and cartilage. Kato et al. microscopically observed changes in the articular cartilage after the injection of chondroitin sulfate ABC endolyase (22,27). They found that the changes were milder than those observed with chymopapain and that it was possible to regenerate chondrocytes after chondroitin sulfate ABC endolyase injection. These findings were observed even when using 4–8 times the dose used clinically. From this perspective, chondroitin sulfate ABC endolyase may be defined as a matrix-specific enzyme that allows normal NP reproduction (22,24,27).
On the other hand, placebo-controlled studies have confirmed that adverse events occurred in seven patients in the chondroitin sulfate ABC endolyase group during 52 weeks of observation (abnormal hepatic function, intervertebral disc protrusion, pneumothorax, hemorrhoids, uterine leiomyoma, lumbar radiculopathy, and back pain), but all of these resolved or improved (11,28).
The injection procedure also plays an important role in safe treatment. Although animal models have shown that epidural injection of chondroitin sulfate ABC endolyase did not injure the surrounding tissues (22), leakage to the epidural space should be minimized to prevent adverse events. Therefore, we used relatively fine needles (22 gauge), which were inserted from the opposite side of the radiculopathy. Furthermore, non-contained IDHs (transligamentous extrusion and sequestration) were excluded from the indication to prevent leakage though tears on the annulus fibrosus. We have already treated approximately 30 patients with chondroitin sulfate ABC endolyase injection, and no adverse event has been observed. To prevent anaphylaxis at the second injection, we provide the patient with a certificate referring chondroitin sulfate ABC endolyase injection and alert the patient to the risk of anaphylaxis.
Conclusions
The use of chondroitin sulfate ABC endolyase in chemonucleolysis is just beginning. Previous studies and clinical trials have confirmed its safety and effectiveness in lumbar IDH treatment. Further clinical experiences may clarify the most appropriate IDH type and patient profile for this treatment. Expansion of the indication for cervical disc herniation is also expected in the near future.
Acknowledgments
We thank Seikagaku Corporation for providing an original image for the 1st illustration. We also thank the operating room staff for their technical assistance, the medical records clerks who helped collect patient data, and the radiology department staff for recording radiographs and MRI data. This work was partly supported by a grant from the Iwai Medical Foundation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Smith L. Enzyme Dissolution of The Nucleus Pulposus In Humans. JAMA 1964;187:137-40. [Crossref] [PubMed]
- Dando PM, Morton DB, Buttle DJ, et al. Quantitative assessment of human proteinases as agents for chemonucleolysis. Spine 1988;13:188-92. [Crossref] [PubMed]
- Takenaka Y, Revel M, Kahan A, et al. Experimental model of disc herniations in rats for study of nucleolytic drugs. Spine (Phila Pa 1976) 1987;12:556-60. [Crossref] [PubMed]
- Wakita S, Shimizu K, Suzuki K, et al. Chemonucleolysis with calpain I in rabbits. Spine 1993;18:159-64. [Crossref] [PubMed]
- Kodama H, Shimizu K, Banno Y, et al. Calpain inhibition by cerebrospinal fluid and effects of calpain on intrathecal nerve tissue. Spine 2002;27:1077-81. [Crossref] [PubMed]
- Antoniou J, Mwale F, Demers CN, et al. Quantitative Magnetic Resonance Imaging of Enzymatically Induced Degradation of the Nucleus Pulposus of Intervertebral Discs. Spine 2006;31:1547-54. [Crossref] [PubMed]
- Sussman BJ, Bromley JW, Gomez JC. Injection of collagenase in the treatment of herniated lumbar disk. Initial clinical report. JAMA 1981;245:730-2. [Crossref] [PubMed]
- Artigas J, Brock M, Mayer HM. Complications following chemonucleolysis with collagenase. J Neurosurg 1984;61:679-85. [Crossref] [PubMed]
- Flanagan N, Smith L. Clinical studies of chemonucleolysis patients with ten- to twenty-year follow-up evaluation. Clin Orthop Relat Res 1986.15-7. [PubMed]
- Tregonning GD, Transfeldt EE, McCulloch JA, et al. Chymopapain versus conventional surgery for lumbar disc herniation. 10-year results of treatment. J Bone Joint Surg Br 1991;73:481-6. [Crossref] [PubMed]
- Matsuyama Y, Chiba K, Iwata H, et al. A multicenter, randomized, double-blind, dose-finding study of condoliase in patients with lumbar disc herniation. J Neurosurg: Spine 2018;28:499-511. [Crossref] [PubMed]
- Takahashi T, Nakayama M, Chimura S, et al. Treatment of canine intervertebral disc displacement with chondroitinase ABC. Spine 1997;22:1435-9; discussion 1446-7. [Crossref] [PubMed]
- Hamai A, Hashimoto N, Mochizuki H, et al. Two distinct chondroitin sulfate ABC lyases. An endoeliminase yielding tetrasaccharides and an exoeliminase preferentially acting on oligosaccharides. J Biol Chem 1997;272:9123-30. [Crossref] [PubMed]
- Dyck P. Paraplegia following chemonucleolysis. A case report and discussion of neurotoxicity. Spine 1985;10:359-62. [Crossref] [PubMed]
- Buchman A, Wright RB, Wichter MD, et al. Hemorrhagic complications after the lumbar injection of chymopapain. Neurosurgery 1985;16:222-4. [Crossref] [PubMed]
- Cusick JF, Ho KC, Schamberg JF. Subarachnoid hemorrhage following chymopapain chemonucleolysis. J Neurosurg 1987;66:775-8. [Crossref] [PubMed]
- Zook BC, Kobrine AI. Effects of collagenase and chymopapain on spinal nerves and intervertebral discs of Cynomolgus monkeys. J Neurosurg 1986;64:474-83. [Crossref] [PubMed]
- Wintermantel E, Emde H, Loew F. Intradiscal collagenase for treatment of lumbar disc herniations. A comparison of clinical results and computed tomography follow-up. Acta Neurochir (Wien) 1985;78:98-104. [Crossref] [PubMed]
- Hedtmann A, Steffen R, Krämer J. Prospective comparative study of intradiscal high-dose and low-dose collagenase versus chymopapain. Spine 1987;12:388-92. [Crossref] [PubMed]
- Yamagata T, Saito H, Habuchi O, et al. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem 1968;243:1523-35. [PubMed]
- Sato N, Shimada M, Nakajima H, et al. Cloning and expression in Escherichia coli of the gene encoding the Proteus vulgaris chondroitin ABC lyase. Appl Microbiol Biotechnol 1994;41:39-46. [Crossref] [PubMed]
- Kato F, Mimatsu K, Iwata H, et al. Comparison of tissue reaction with chondroitinase ABC and chymopapain in rabbits as the basis of clinical application in chemonucleolysis. Clin Orthop Relat Res 1993.294-302. [PubMed]
- Sugimura T, Kato F, Mimatsu K, et al. Experimental chemonucleolysis with chondroitinase ABC in monkeys. Spine 1996;21:161-5. [Crossref] [PubMed]
- Chiba K, Masuda K, Andersson GB, et al. Matrix replenishment by intervertebral disc cells after chemonucleolysis in vitro with chondroitinase ABC and chymopapain. Spine J 2007;7:694-700. [Crossref] [PubMed]
- Olmarker K, Danielsen N, Nannmark U, et al. Microvascular effects of chondroitinase ABC and chymopapain. An in vivo experimental study on hamsters and rabbits. Clin Orthop Relat Res 1990.274-9. [PubMed]
- Olmarker K, Strömberg J, Blomquist J, et al. Chondroitinase ABC (pharmaceutical grade) for chemonucleolysis. Functional and structural evaluation after local application on intraspinal nerve structures and blood vessels. Spine 1996;21:1952-6. [Crossref] [PubMed]
- Kato F, Iwata H, Mimatsu K, et al. Experimental chemonucleolysis with chondroitinase ABC. Clin Orthop Relat Res 1990.301-8. [PubMed]
- Chiba K, Matsuyama Y, Seo T, et al. Condoliase for the Treatment of Lumbar Disc Herniation: A Randomized Controlled Trial. Spine (Phila Pa 1976) 2018;43:E869-76. [PubMed]
- Brandner ME. Normal values of the vertebral body and intervertebral disk index in adults. The American journal of roentgenology, radium therapy, and nuclear medicine 1972;114:411-4. [Crossref] [PubMed]


