
Complication avoidance in minimally invasive spinal surgery
Introduction
A variety of minimally invasive surgical (MIS) techniques have been developed to reduce the morbidity associated with traditional open spinal surgery (1). Because MIS procedures involve reduced exposure, narrow surgical corridors, and/or novel approaches to minimize damage to surrounding tissues, they introduce a unique set of potential complications. The literature suggests that surgeons less familiar with these techniques exhibit elevated complication rates until the learning curve has been overcome (2). However, knowledge of these risks as well as strategies to avoid them can allow surgeons to perform MIS spinal surgery safely and reliably (3-5). This goal of the present paper therefore is to identify complications specific to MIS techniques and provide tactics for their prevention. This is accomplished via a review of the relevant literature combined with the authors’ personal experiences.
Posterior cervical foraminotomy (PCF)
Decision making and pre-operative planning
PCF is best suited for foraminal pathology rather than central neural compression. It should be avoided in patients presenting with axial neck pain in the absence of neurologic symptoms, as it is unlikely to provide relief in this setting. Gross cervical instability or significant kyphosis limits the effectiveness of posterior decompression may exacerbate deformity and/or instability. Extensive ventral neural compression will not be adequately addressed by PCF (3).
Pre-operative imaging, including anteroposterior (AP), lateral, flexion-extension radiographs as well as magnetic resonance imaging (MRI) or computed tomography (CT) myelogram should be used to confirm that the pathology is amenable to PCF and to rule out instability or kyphotic deformity.
Positioning and set up
Pre-operative cervical spine range of motion should be examined and should not be exceeded in the operating room during induction of anesthesia or patient positioning to avoid neurologic damage (6). If intubation via standard techniques would require neck extension beyond that tolerated by the patient pre-operatively, fiberoptic intubation should be performed.
Sitting and prone positioning have been described (3,7). All boney prominences should be well padded to avoid pressure injury. The eyes should be free of external pressure and protected to avoid chemical injury from preparation solutions (8). Mayfield three-point or an analogous rigid head fixation device is used when the patient is in the sitting position and by some surgeons in the prone position as well. While outside the scope of this article, the surgeon should be comfortable with use of such devices as improper pin placement can result in damage to underlying structures or insufficient purchase on the skull. If prone positioning is utilized, the patient’s head is at least slightly elevated to decrease intraoperative bleeding. The cervical spine is placed in mild flexion but neutral rotation to facilitate access (3,7).
Radiographic landmarks
It is important that the operative level can be adequately visualized on fluoroscopy, and this should be confirmed prior to prepping and draping. Obtaining an acceptable lateral view may prove difficult at lower cervical levels where the shoulders may obscure cervical anatomy. Should this be the case, the shoulders can be taped down. However, excessive tension can produce brachial plexopathies and should be avoided (9).
Surgical technique
The incision should be placed in line with the disc space as confirmed on lateral fluoroscopy and approximately 0.5–1 cm lateral to midline. Placing the incision too far laterally necessitates a more medially-directed trajectory and makes it more likely that the surgeon will encounter the central canal than the neuroforamen.
The fascial incision should be made just lateral to the spinous process, preserving the midline ligamentous structures to maintain stability. It is important to incise completely through the fascia so that dilators can be passed without undue force (3,7).
During dilation for and placement of the tubular retractor, the goal is to dock on the medial aspect of the lateral mass, parallel to the disc space. The authors do not use guide wires because of the risk of inadvertent entry into the spinal canal. An initial dilator can be carefully guided onto the lateral mass using finger palpation of the boney structures. Avoid a too-medial trajectory, and do not use undue force during passage of the dilators as it is possible to enter the intralaminar window with potentially disastrous neurologic consequences. Sliding laterally off the facet joint could result in vertebral artery injury (3); the lamina and facet joints in the upper cervical spine tend to slope more laterally so extra care should be taken at these levels (7). Fluoroscopic imaging should be used periodically during dilator and retractor placement to confirm appropriate level, docking position and trajectory.
The microscope is usually required to provide optimal visualization. While the medial 1/3rd of the facet joint will be removed to access and decompress the foramen, the remainder of the capsule and joint should be carefully preserved to avoid iatrogenic instability. A least 50% of the facet joint should be preserved to maintain biomechanical integrity (10). Given the more limited view via the tubular retractor, it is imperative that the surgeon understands the local anatomy before removing bone or facet capsule. This can be facilitated by fluoroscopic imaging and by “wanding” the retractor as necessary. It may also help to begin exposure on the lateral edges of the superior and inferior lamina, following them to the interlaminar “V”. This site reflects the junction of the lamina and lateral mass. The authors widen the interlaminar “V” which provides access to the lateral spinal canal and the entrance to the foramen. The foraminotomy-discectomy then proceeds in a similar fashion to its open counterpart.
In the event of an incidental durotomy, direct suture repair is often difficult within the confined field created by a tubular retractor. Small defects may be addressed with some combination of muscle or fat grafts, gelatin or thrombin-based topical hemostatic agents, dural substitute, and dural sealant (3). Care should be taken to avoid leaving large volumes of these materials in close proximity to the neural elements to avoid neural compression. Larger durotomies should be directly repaired with suture. Minimally invasive dural repair sets contain instruments designed to be used down tubular retractor systems. However, inability to perform adequate repair via the tubular retractor should prompt the surgeon to convert to an open approach.
When removing the tubular retractor after completion of the decompression, it is advisable to withdraw it slowly while simultaneously coagulating any sites of muscle bleeding to prevent post-operative epidural hematoma (7). Fascial closure can be facilitated by the use of heavy suture on a urology needle (e.g., UR-6)—these needles have a smaller radius of curvature and are therefore easier to pass through a small incision (7).
Post-operative management
Post-operative management is similar to that of open PCF.
Lateral lumbar interbody fusion (LLIF)
Decision making and pre-operative planning
Pre-operative planning for LLIF involves assessment of anatomic structures uniquely relevant to this procedure. The level of the iliac crests on the AP and lateral radiograph should be noted. High-riding iliac crests may impede access to L4–5 and may be a contraindication to this approach. The locations of the major vascular structures [aorta and inferior vena cava (IVC) cranially and iliac vessels rostrally] should be assessed on axial MRI. The position and morphology of the psoas muscles should also be noted on axial MRI cuts at the level of the disc. At the L4–5 level, branches of the lumbar plexus are situated more anteriorly (11). Teardrop psoas morphology has been associated with a more anterior location of the lumbar plexus as well as more posterolateral position of the major vessels, which may place these neurovascular structures at risk (12). Failure to recognize these factors pre-operatively and modify the operative plan accordingly could result in vascular or neurologic injury. Additionally, approaching the spine from the side of prior retroperitoneal disease or surgery (e.g., nephrectomy) should be avoided.
In the setting of deformity, it is important to understand the expected degree of correction from an LLIF to prevent post-operative regional or global malalignment. This technique has demonstrated excellent ability to correct coronal deformity (13-15) but has been shown to produce only modest improvements in lumbar lordosis and pelvic tilt (15,16). If more substantial sagittal correction is desired, lateral techniques such as anterior column realignment (ACR) via anterior longitudinal ligament (ALL) release and placement of a hyperlordotic cage should be considered. ACR can be expected to produce 14° of segmental lordosis, which can be increased to 21–27° with the addition of posterior facetectomies and up to 30° with facetectomies, spinous process resection, and hyperlordotic cage placement (17).
If performing LLIF in patients with spinal instability or significant coronal or sagittal imbalance, supplemental posterior fixation is advisable. Stand-alone LLIF should also be avoided in patients with low bone mineral density to reduce the risk of subsidence.
Positioning/set up
The patient is placed in the lateral position on an operating table, which has been oriented to allow for unimpeded access for the C-arm. This is essential given the importance of image guidance during this procedure.
All boney prominences should be well padded to prevent pressure injuries, and an axillary roll is placed to protect the brachial plexus. The hips are flexed to 45° and the knees to 90° to reduce tension on the psoas muscle and lumbar plexus.
The operative level should be located at the break in the table, which can be flexed to improve access as the ilium or ribs may obstruct the approach to L4–5 or upper lumbar levels, respectively. However, table flexion should be limited to that necessary to safely access the level of interest as this jack-knife position alone has been associated with post-operative neuropraxia (18).
Silk tape is used to firmly hold the patient in this position, and a supplemental kidney post can be placed behind the pelvis. Secure positioning is essential as this procedure depends upon perfect fluoroscopic imaging to be performed safety.
Patient positioning and/or the operating table should be adjusted until perfect AP and lateral fluoroscopy views can be obtained with the fluoroscopy beam parallel and perpendicular to the floor, respectively. This allows the surgeon to avoid straying too far anterior or posterior, which would place neurovascular structures at risk.
Radiographic landmarks
The quality of the AP and lateral fluoroscopy views should be confirmed after the patient is prepped and draped to confirm that there has been no change in positioning. If necessary, the table should be adjusted to ensure perfect vertebral endplate views with no rotation.
Surgical technique
During the approach, the abdominal wall muscles should be bluntly split in line with their fibers, avoiding monopolar electrocautery to prevent damage the abdominal wall innervation (e.g., iliohypogastric and subcostal nerves) and the potential for abdominal wall paresis and pseudohernia (19). After entering the retroperitoneal space, the surgeon should sweep the peritoneal contents anteriorly to avoid ensnaring them during the process of retractor docking and placement. Depending on the surgical level, injuries to bowel, kidney, diaphragm, and major vascular structures are possible (13,14,20,21). All dilators and the retractor itself should be guided down to the disc space ensuring that the surrounding tissues are free. An auxiliary posterior incision, placed just lateral to the ipsilateral paraspinal muscle can be used to help with this process.
The use of directional, real-time electromyography (EMG) is critical to minimize the risk of motor nerve injury (22). This is especially pertinent at L4–5 due to the more anterior location of the intra-psoas lumbar plexus at this level (11). Unacceptably low EMG values should prompt the surgeon to reposition the dilators or retractor. Docking in a slightly more anterior position will often result in improved EMG values. If working in the anterior 1/3rd of the disc space, care should be taken to avoid inadvertent sectioning of the ALL and injury to anterior vascular structures. If a safe docking site cannot be identified, the lateral procedure should be aborted. Once the retractor is docked and opened, the surgical field should be visually inspected to confirm that no crossing structures overly the intervertebral space. EMG stimulation of the surgical field should be performed.
During the intra-discal work, all instruments as well as the final implant should be directly visualized entering the disc space by the surgeon. Fluoroscopy should be used to avoid plunging past the far side of the vertebral body, which could cause injury to contralateral structures.
The integrity of the endplates should be carefully maintained during disc space preparation and cage insertion. Violation of these structures increases the risk of subsidence which may result in pseudarthrosis, loss of indirect decompression, and loss of lordosis. Optional slides are available with many lateral fusion systems and may minimize endplate damage while inserting the final implant. Avoid the use of an excessively tall cage in an effort to maximize indirect decompression as doing so may result in endplate damage, especially in patients with low bone mineral density (23). The use of cages with wider footprints appears to be protective against subsidence, with published subsidence rates of 14.1% vs. 1.9% with 18- and 22-mm wide cages respectively (24).
While every surgery should be performed deliberately and methodically, LLIF must also be performed efficiently. Longer intra-psoas retraction times have been associated with elevated rates of lumbar plexus-related neurologic deficits (25,26). Uribe and colleagues reported significantly longer retractor times in patients with symptomatic post-operative neuropraxias compared to those without (32.3 vs. 22.6 minutes) (25). If it appears that retractor times will exceed 25–30 minutes, the surgeon should consider temporarily collapsing the retractor to take stretch and pressure off the lumbar plexus.
Post-operative abdominal wall hernia is an uncommon complication of lateral approaches but one that may necessitate return to the operating room for repair (5,15,27). These hernias may be the result of inadequate fascial closure at the time of the index surgery and might therefore be prevented by meticulous attention to this portion of the procedure. Robust closure of the transversalis fascial should be performed, avoiding the underlying muscle layers as damage to traversing nerves contained within them can result in abdominal wall paresis and pseudohernia (27).
Post-operative management
Post-operative ileus has been documented in 7% of patients after LLIF and has been associated with a history of gastroesophageal reflux disease, placement of posterior instrumentation, and surgery at the L1–2 level (28). Because unrecognized ileus may progress to Ogilvie’s syndrome with associated mortality rates exceeding 50%, close monitoring of post-operative bowel function is essential (29). Patients with suspected ileus should be appropriately worked up and managed.
Consider bracing the patient post-operatively to avoid subsidence or cage migration, especially if performing a stand-alone LLIF or in patients with low bone mineral density.
Oblique lumbar interbody fusion (OLIF)
Decision making and pre-operative planning
OLIF is a modification of the LLIF technique developed to avoid traversing the psoas muscle, theoretically reducing the incidence of psoas and lumbar plexus injury. To achieve this, an oblique, anterolateral course is taken to the spine, and the entry corridor to the disc space lies between the anterior border of the psoas muscle and the lateral border of either the aorta or the left common iliac artery. However, much of the subsequent surgical technique is similar to LLIF. Considerations specific to OLIF are discussed below.
A left-sided approach is typically performed as the major arteries more robust than their corresponding veins. This is even more pertinent than in LLIF as the more anterior trajectory inherent to OLIF places the surgical window closer to these vessels. While a right-sided approach is possible, it is inadvisable except by surgeons who are highly experienced with this technique (30).
The distance between the anterior border of the psoas the lateral border of the artery should be measured on the pre-operative axial imaging and should be 1 cm or larger to reduce the risk of vascular damage or retraction-related injury to the psoas or lumbar plexus (31). Otherwise, another technique for lumbar fusion should be utilized. The presence of teardrop psoas morphology at L4–5 is associated with anterior migration of the psoas and posterolateral location of the iliac vasculature, properties that may narrow the approach corridor and increase the risk of vascular injury (12).
Positioning/set up
Patient positioning is analogous to that described in the above section on LLIF with similar considerations for avoiding complications. The patient is positioned in the lateral decubitus position with the knees and hips flexed. The operative level should be situated at the table break, which can be flexed slightly to improve access. All boney prominences should be well padded. The patient is then secured to the table using adhesive tape with or without a supplemental post.
Radiographic landmarks
Perfect AP and lateral views are required as in the LLIF procedure.
Surgical technique
Given the more anterior skin incision and oblique approach trajectory of the OLIF procedure, peritoneal lacerations have been reported significantly more frequently with OLIF than with LLIF (0.8% vs. 0.05%, P=0.001) (32). The transversalis fascia should therefore be penetrated as far laterally as possible with an oblique, posteriorly directed trajectory to avoid inadvertent entry into the peritoneal space (30,33). Blunt dissection through the retroperitoneal space is performed until the psoas muscle is reached. The anterior border of the psoas can be gently mobilized posteriorly using blunt finger dissection to increase the size of the surgical corridor and distance from the major vascular structures (33). Compared to LLIF, the great vessels, renal vessels and segmental arteries are in closer proximity in OLIF (31,34) and may be at elevated risk of injury. The L4 and L5 segmental arteries, in particular, may run in close proximity to or even across the intervertebral disc within the OLIF surgical corridor—scrutiny of pre-operative imaging, use of a blunt cannulated dilator (rather than a sharp guidewire) when initially finding the disc space, and avoidance of pin fixation of the retractor at these levels may reduce the risk of vascular injury (33,34).
The sympathetic chain runs anterior to the psoas along the anterolateral vertebral bodies and may be damaged during this procedure, causing vasodilation, swelling, discoloration, anhidrosis and dysesthesias in the ipsilateral lower extremity (35). Kim et al. described this complication in 13.5% of the patients in their OLIF series (36). Strategies for avoiding this complication include careful dissection with a focus on identifying the sympathetic chain if it lies within the surgical corridor; it may be mobilized anteriorly if necessary, and the retractor should be placed posterior to it (33,37). Ureteral injury may also occur during OLIF (32). This risk can be minimized by visually confirming that all retroperitoneal fatty tissue is bluntly mobilized and retracted off of the disc space prior to discectomy (33).
While the retractor is docked and opened obliquely over the anterolateral aspect of the disc space, disc preparation, trialing, and implant insertion should all be performed orthogonally to the disc space. This entails the use of angled instruments and/or pushing the instrument handle posteriorly while working. Failure to do so can result in violation or impingement of posterior neural structures because insertion in line with the approach trajectory places the contralateral foramen and exiting nerve root at risk. Kraiwattanapong et al. reported a case of contralateral nerve root compression requiring return to the operating room as a result of posterior and deep positioning of an OLIF cage (38). At least one instance of a large, irreparable ventral dural tear has also been reported (39). Occasional fluoroscopic checks can help avoid working too far posteriorly in the disc space.
Post-operative management
Post-operative protocols are similar to that described in the above LLIF section.
Lumbar decompression (microdiscectomy and laminectomy)
Decision making and pre-operative planning
The AP radiograph and axial imaging should be studied for the presence of spina bifida occulta and/or prior laminectomy defects before every procedure. Failure to appreciate defects in the posterior arch could result in inadvertent dural violation and neurologic injury during retractor placement or exposure.
The approach is typically performed from the side of greatest pathology by the authors. In patients with central or bilateral pathology, it is advisable to approach from the most symptomatic side. Decompression from either side can be justified if both sides are equally affected.
Positioning/set up
The operating room is arranged such that the surgeon stands on the side of the approach, and the fluoroscopy unit enters from the contralateral side (40). Other than placement of an attachment clamp onto the table frame for the tubular retractor arm, positioning is similar to that of a standard lumbar decompression.
Radiographic landmarks
Incision localization can be performed on AP or lateral fluoroscopy. Regardless of the preferred view, a perfectly orthogonal image should be obtained. On the AP, this entails crisp endplates at the level of interest, symmetric pedicles, and a midline spinous process. The authors also ensure that a clear view of the interlaminar window is obtained on the AP projection. On the lateral, endplates should be crisp and pedicles perfectly superimposed. Utilizing suboptimal imaging can lead to improper incision and retractor placement, added technical difficulty during the procedure, and even damage to surrounding structures.
Surgical technique
A vertical incision is made approximately one cm lateral to midline. Given the slightly oblique trajectory of the retractor, the incision is made slightly more laterally in larger patients (40) or when contralateral decompression is required. Failure to make this adjustment can make it harder to work medially.
The initial dilator should be carefully directed onto the inferior border of the lamina medial to the facet joint at the spinolaminar junction on the side of the approach and the location confirmed on fluoroscopy. Sequential dilation is performed, and the tubular retractor placed (Figure 1). Care should be taken to ensure the leading edges of all dilators and the final retractor are in contact with bone (i.e., lamina).
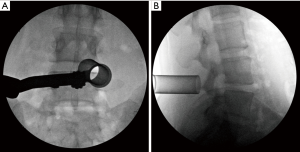
Constant, gentle downward pressure should by applied during dilation and retractor placement to prevent paraspinal muscle from entering the tube (40). Lack of attention to this step impairs visualization and requires removal of healthy muscle, which may contribute to post-operative bleeding and pain.
In morbidly obese patients, standard tubular retractors may not be sufficiently long. While custom, extra-long retractors can be procured, working through such long tubes becomes increasingly difficult. One strategy to address this issue is to extend the skin incision, retract the superficial tissues with a self-retaining retractor, and then dock the tubular retractor within this mini-open incision.
While a larger diameter tube provides more working room and maneuverability, wider tubes do have disadvantages. Beyond requiring larger incisions, it may be difficult to dock wider tubes flush against the lamina in the presence of facet hypertrophy or in the narrower upper lumbar spine.
It is imperative that the surgeon maintain sufficient pars and facet joint (>50%) to avoid iatrogenic instability (41) and/or pars fracture. Despite the confined view of a tubular retractor, the surgeon must be acutely aware of the surrounding anatomy. The upslope of the base of the spinous process medially and the start of the facet joint laterally should be visualized. The caudal edge of the cephalad lamina should be identified to facilitate entry into the spinal canal. Additionally, direct palpation or visualization of the lateral border of the pars can help provide orientation and guide osseous resection.
If performing a bilateral decompression via a unilateral hemilaminotomy, it is best to perform ipsilateral and contralateral osseous resection using a bur before removing ligamentum flavum, which serves as a protective barrier during bone removal. This will partially decompress the space, reducing the risk of incidental durotomy and/or retraction-related neurologic deficits during subsequent steps. A side-cutting burr is used for the majority of bone resection (as the smooth tip is less likely to damage underlying tissue), which is then completed with a Kerrison punch. When working to the contralateral side, follow the ligamentum as it slopes ventrally and laterally, removing the bone immediately overlying it.
As with any tubular-based procedure, direct suture repair of a dural tear can be difficult via the tubular retractor (see PCF section above). The surgeon should convert to an open approach if unable to achieve adequate repair through the tube.
Meticulous hemostasis with attention to epidural veins, cut boney edges, and deep and superficial soft tissues is especially important in tubular-based surgery as the muscle-preserving nature of these procedures leaves minimal dead space to accommodate post-operative hematoma. Less post-operative bleeding can therefore be tolerated before pressure is transmitted to the neural elements.
Post-operative management
Patients can follow similar post-operative protocols regardless of whether a tubular versus traditional open approach was utilized.
Percutaneous pedicle screw placement
Decision making and pre-operative planning
Radiographs and advanced imaging should be inspected for defects in the posterior elements, which may be due to congenital or iatrogenic factors. Additionally, projected screw length and diameter can be planned in advance on pre-operative imaging to avoid pedicle or anterior cortex violation.
Positioning/set up
Given the lack of direct visualization inherent to percutaneous pedicle screw placement, the surgeon relies on a keen understanding of anatomy, tactile feedback, and imaging. The latter can involve fluoroscopy (one or two C-arms), navigation, or robotics. Each modality has unique benefits and drawbacks, which are beyond the scope of this article. Regardless, the surgeon should be well versed in the set up required for the selected technique. Because navigation and robotics are not available at every center, the discussion herein will focus on screw placement under fluoroscopic guidance.
Radiographic landmarks
Approximately 50% of spine surgeons report performing at least one wrong-level surgery over the course of their careers (42,43). Careful attention to radiographic level localization is especially important in minimally invasive and percutaneous techniques where open landmarks are not available for confirmation. Counting up or down from a known radiographic landmark (e.g., sacrum) can help reduce error. In the thoracic spine, where localization can be especially difficult, strategies for improving reliability include: (I) placing sterile spinal needles into osseous landmarks under fluoroscopy starting at caudal levels and working successively cephalad (44); (II) having interventional radiology place radiopaque markers into the boney structures of specific vertebrae pre-operatively (45,46), or (III) using intra-operative navigation.
Once the correct level has been identified, perfect orthogonal images are required to safely and reliably place percutaneous screws. Relying on suboptimal images reduce accuracy, which can lead to improperly placed implants and damage to surrounding neurovascular structures.
Surgical technique
Incisions are planned based on AP fluoroscopy—they are usually placed approximately 5 mm lateral to the lateral border of the pedicle but should be made further lateral in obese patients given the oblique insertion trajectory. Failure to account for this fact may result in difficulty sufficiently medializing pedicle screws and resultant lateral blowout.
Throughout the procedure, the surgeon should not rely solely on image guidance but should also employ tactile feedback, palpating boney landmarks for the start point and feeling cancellous bone as the Jamshidi is advanced down the pedicle. It is important to stop and reassess if imaging findings do not correlate with physical feedback. Avoid multiple passes with the Jamshidi to minimize post-operative pain as well as potential damage to surrounding structures.
When advancing the Jamshidi through the pedicle, it is important that the tip of the instrument does not cross the medial border of the pedicle on the AP projection until the pedicle has been traversed and the tip lies within the vertebral body on the lateral view. Crossing the medial border prior to entry into the vertebral body indicates a medial wall violation with potential damage to neurologic structures. Once the Jamshidi has been appropriately positioned, guidewires should be inserted such they traverse approximately 50% of the vertebral body—this leaves sufficient room for a small amount of anterior migration of the wire without penetrating the anterior vertebral body cortex. An oblique, owl’s eye fluoroscopy view directly down the pedicle can be taken once the guidewire has been placed to confirm that it passes within the pedicle.
During tapping and screw insertion, periodic lateral fluoroscopy shots should be taken to ensure that the guidewires have not advanced past the anterior wall of the vertebral body, potentially injuring surrounding vascular structures and viscera. The cannulated taps and screws should always be introduced colinearly with the guidewires to avoid guidewire kinking and the potential for breakage. Dynamic EMG can be used during screw placement to provide real-time feedback on screw proximity to neurologic structures (47).
Once the rod is inserted and held with set screws, confirm on fluoroscopic imaging (and with direct visualization if possible) that the rod has not bypassed any of the screw heads. Failure to recognize and address this issue in the operating room will result in a suboptimal construct. Do not break off screw tabs prior to such confirmation as it will make any necessary subsequent rod repositioning unnecessarily challenging.
Post-operative management
No specific restrictions or post-operative concerns are unique to this technique as compared with its open counterpart.
Transforaminal lumbar interbody fusion (TLIF)
Decision making and pre-operative planning
While MIS and open TLIF have been shown to produce similar improvements in lumbar lordosis (48,49), these gains are relatively modest in nature, especially when compared to those achieved with anterior lumbar interbody fusion (ALIF) or LLIF (50,51). Watkins et al. reported significant increases in lumbar lordosis with ALIF and LLIF (4.5° and 2.2°, respectively) but not with TLIF (50). This technique is therefore less suited to scenarios necessitating substantial sagittal correction. Furthermore, using an MIS transforaminal approach to access and mobilize a severely collapsed disc space or in the setting of high-grade spondylolisthesis may prove technically difficult.
Once the decision is made to perform an MIS TLIF, pre-operative imaging should be reviewed for the presence of pre-existing defects in the posterior elements. The side of the approach is dictated by the location of the pathology.
Positioning/set up
Set up and positioning is as described in the preceding section on lumbar decompression.
Radiographic landmarks
Perfect AP and lateral fluoroscopy views, as described above, are imperative to execute this procedure safely and reliably.
Surgical technique
The pedicle screw placement incisions are connected on the side of the TLIF approach, and the tubular retractor inserted through this opening. A standard hemilaminotomy should be performed and the disc space identified prior to removal of the facet joint as the disc space serves as a guide for facet resection—bone overlying the disc space should be excised. While it is important to skeletonize the cranial aspect of the caudal pedicle to maximize the working window, removing the facet joint without first becoming oriented to the location of the disc space increases the risk of inadvertent pedicle violation with a bur. Additionally, the authors believe it is beneficial to maintain the pars cranial to the disc space as this structure protects the underlying exiting nerve root during disc preparation and cage insertion.
Inserting pedicle screws and a rod held loosely with set screws on the side contralateral to the TLIF prior to disc preparation allows the surgeon to maintain paddle-based distraction by locking down the rod once the paddle is in place. This will enlarge the working window to facilitate disc space preparation (i.e., more thorough discectomy with less likelihood of endplate violation) and maximize cage height.
Thorough disc space preparation is especially important during MIS TLIF when the sole location of fusion is the interbody space (i.e., unless supplemental posterolateral or facet fusion has been performed). Great care should be taken during discectomy, however, because violation of the ALL can result in damage to surrounding structures (e.g., aorta) with life threatening consequences (52). Additionally, an intact ALL is necessary to contain bone graft and the interbody cage. Direct visualization and periodic lateral fluoroscopy should be utilized during disc space preparation, bone graft insertion, and cage placement to ensure that nothing exits the disc space anteriorly.
Midline cage positioning to produce symmetric distraction requires significant medialization during insertion. The authors initiate cage placement with a trajectory away from the medial neural elements. Once the leading edge of the cage is secure within the disc space, medialization begins. To avoid placing the cage too far laterally, the tubular retractor arm should be loosened such that the insertion trajectory can be medialized without limitation from the retractor.
Post-operative management
Post-operative management and concerns are similar to that of open TLIF.
Discussion
Minimally invasive spine surgery bears many advantages but comes with a unique set of potential pitfalls—many of which may be minimized using the techniques described above. In addition to reading on this topic, surgeons interested in incorporating MIS techniques into their operative repertoire should consider participating in cadaveric workshops, operating with experienced surgeons, and carefully selecting their early cases. In doing so, they can mount the learning curve and perform these procedures in a safe and reliable manner.
Acknowledgments
None.
Footnote
Conflicts of Interest: Dr. Phillips reports the following relationships: Royalties: Nuvasive, Medtronic, Stryker; Consulting Payments: Nuvasive, SI Bone, Stryker; Stock/Options: Nuvasive, SI-Bone, Theracell (BOD), Surgio (BOD), Edge Surgical (BOD), Providence, Mainstay, Carbofix. Dr. Derman has no conflicts of interest to declare.
References
- Oppenheimer JH, DeCastro I, McDonnell DE. Minimally invasive spine technology and minimally invasive spine surgery: a historical review. Neurosurg Focus 2009;27:E9. [Crossref] [PubMed]
- Sclafani JA, Kim CW. Complications associated with the initial learning curve of minimally invasive spine surgery: a systematic review. Clin Orthop 2014;472:1711-7. [Crossref] [PubMed]
- Gala VC, O'Toole JE, Voyadzis JM, et al. Posterior minimally invasive approaches for the cervical spine. Orthop Clin North Am 2007;38:339-49. abstract v. [Crossref] [PubMed]
- Goldstein CL, Phillips FM, Rampersaud YR. Comparative Effectiveness and Economic Evaluations of Open Versus Minimally Invasive Posterior or Transforaminal Lumbar Interbody Fusion: A Systematic Review. Spine 2016;41 Suppl 8:S74-89. [PubMed]
- Rodgers WB, Gerber EJ, Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: an analysis of 600 cases. Spine 2011;36:26-32. [Crossref] [PubMed]
- Durga P, Sahu BP. Neurological deterioration during intubation in cervical spine disorders. Indian J Anaesth 2014;58:684-92. [Crossref] [PubMed]
- Ross DA, Bridges KJ. Technique of Minimally Invasive Cervical Foraminotomy. Oper Neurosurg (Hagerstown) 2017;13:693-701. [Crossref] [PubMed]
- Bever GJ, Brodie FL, Hwang DG. Corneal Injury from Presurgical Chlorhexidine Skin Preparation. World Neurosurg 2016;96:610.e1-610.e4. [Crossref] [PubMed]
- Jahangiri FR, Holmberg A, Vega-Bermudez F, et al. Preventing position-related brachial plexus injury with intraoperative somatosensory evoked potentials and transcranial electrical motor evoked potentials during anterior cervical spine surgery. Am J Electroneurodiagnostic Technol 2011;51:198-205. [Crossref] [PubMed]
- Raynor RB, Pugh J, Shapiro I. Cervical facetectomy and its effect on spine strength. J Neurosurg 1985;63:278-282. [Crossref] [PubMed]
- Park DK, Lee MJ, Lin EL, et al. The relationship of intrapsoas nerves during a transpsoas approach to the lumbar spine: anatomic study. J Spinal Disord Tech 2010;23:223-8. [Crossref] [PubMed]
- Louie PK, Narain AS, Hijji FY, et al. Radiographic Analysis of Psoas Morphology and its Association With Neurovascular Structures at L4-5 With Reference to Lateral Approaches. Spine 2017;42:E1386-92. [Crossref] [PubMed]
- Tormenti MJ, Maserati MB, Bonfield CM, et al. Complications and radiographic correction in adult scoliosis following combined transpsoas extreme lateral interbody fusion and posterior pedicle screw instrumentation. Neurosurg Focus 2010;28:E7. [Crossref] [PubMed]
- Wang MY, Mummaneni PV. Minimally invasive surgery for thoracolumbar spinal deformity: initial clinical experience with clinical and radiographic outcomes. Neurosurg Focus 2010;28:E9. [Crossref] [PubMed]
- Caputo AM, Michael KW, Chapman TM, et al. Extreme lateral interbody fusion for the treatment of adult degenerative scoliosis. J Clin Neurosci 2013;20:1558-63. [Crossref] [PubMed]
- Le TV, Vivas AC, Dakwar E, et al. The effect of the retroperitoneal transpsoas minimally invasive lateral interbody fusion on segmental and regional lumbar lordosis. ScientificWorldJournal 2012;2012:516706. [Crossref] [PubMed]
- Uribe JS, Harris JE, Beckman JM, et al. Finite element analysis of lordosis restoration with anterior longitudinal ligament release and lateral hyperlordotic cage placement. Eur Spine J 2015;24 Suppl 3:420-6. [Crossref] [PubMed]
- Molinares DM, Davis TT, Fung DA, et al. Is the lateral jack-knife position responsible for cases of transient neurapraxia? J Neurosurg Spine 2016;24:189-96. [Crossref] [PubMed]
- Plata-Bello J, Roldan H, Brage L, et al. Delayed Abdominal Pseudohernia in Young Patient After Lateral Lumbar Interbody Fusion Procedure: Case Report. World Neurosurg 2016;91:671.e13-6. [Crossref] [PubMed]
- Isaacs RE, Hyde J, Goodrich JA, et al. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: perioperative outcomes and complications. Spine (Phila Pa 1976) 2010;35:S322-30. [Crossref] [PubMed]
- Aichmair A, Fantini GA, Garvin S, et al. Aortic perforation during lateral lumbar interbody fusion. J Spinal Disord Tech 2015;28:71-5. [Crossref] [PubMed]
- Uribe JS, Vale FL, Dakwar E. Electromyographic monitoring and its anatomical implications in minimally invasive spine surgery. Spine 2010;35:S368-74. [Crossref] [PubMed]
- Satake K, Kanemura T, Yamaguchi H, et al. Predisposing Factors for Intraoperative Endplate Injury of Extreme Lateral Interbody Fusion. Asian Spine J 2016;10:907-14. [Crossref] [PubMed]
- Le TV, Baaj AA, Dakwar E, et al. Subsidence of polyetheretherketone intervertebral cages in minimally invasive lateral retroperitoneal transpsoas lumbar interbody fusion. Spine 2012;37:1268-73. [Crossref] [PubMed]
- Uribe JS, Isaacs RE, Youssef JA, et al. Can triggered electromyography monitoring throughout retraction predict postoperative symptomatic neuropraxia after XLIF? Results from a prospective multicenter trial. Eur Spine J 2015;24 Suppl 3:378-85. [Crossref] [PubMed]
- Pumberger M, Hughes AP, Huang RR, et al. Neurologic deficit following lateral lumbar interbody fusion. Eur Spine J 2012;21:1192-9. [Crossref] [PubMed]
- Januszewski J, Vivas AC, Uribe JS. Limitations and complications of minimally invasive spinal surgery in adult deformity. Ann Transl Med 2018;6:109. [Crossref] [PubMed]
- Al Maaieh MA, Du JY, Aichmair A, et al. Multivariate analysis on risk factors for postoperative ileus after lateral lumbar interbody fusion. Spine 2014;39:688-94. [Crossref] [PubMed]
- Januszewski J, Keem SK, Smith W, et al. The Potentially Fatal Ogilvie’s Syndrome in Lateral Transpsoas Access Surgery: A Multi-Institutional Experience with 2930 Patients. World Neurosurg 2017;99:302-7. [Crossref] [PubMed]
- Woods KR, Billys JB, Hynes RA. Technical description of oblique lateral interbody fusion at L1-L5 (OLIF25) and at L5-S1 (OLIF51) and evaluation of complication and fusion rates. Spine J 2017;17:545-53. [Crossref] [PubMed]
- Liu L, Liang Y, Zhang H, et al. Imaging Anatomical Research on the Operative Windows of Oblique Lumbar Interbody Fusion. PloS One 2016;11:e0163452. [Crossref] [PubMed]
- Fujibayashi S, Kawakami N, Asazuma T, et al. Complications Associated With Lateral Interbody Fusion: Nationwide Survey of 2998 Cases During the First 2 Years of Its Use in Japan. Spine 2017;42:1478-84. [Crossref] [PubMed]
- Quillo-Olvera J, Lin GX, Jo HJ, et al. Complications on minimally invasive oblique lumbar interbody fusion at L2-L5 levels: a review of the literature and surgical strategies. Ann Transl Med 2018;6:101. [Crossref] [PubMed]
- Orita S, Inage K, Sainoh T, et al. Lower Lumbar Segmental Arteries Can Intersect Over the Intervertebral Disc in the Oblique Lateral Interbody Fusion Approach With a Risk for Arterial Injury: Radiological Analysis of Lumbar Segmental Arteries by Using Magnetic Resonance Imaging. Spine 2017;42:135-42. [Crossref] [PubMed]
- Mahatthanatrakul A, Itthipanichpong T, Ratanakornphan C, et al. Relation of lumbar sympathetic chain to the open corridor of retroperitoneal oblique approach to lumbar spine: an MRI study. Eur Spine J 2019;28:829-34. [Crossref] [PubMed]
- Kim JS, Choi WS, Sung JH. 314 Minimally Invasive Oblique Lateral Interbody Fusion for L4-5: Clinical Outcomes and Perioperative Complications. Neurosurgery 2016;63:190-1. [Crossref]
- Mehren C, Mayer HM, Zandanell C, et al. The Oblique Anterolateral Approach to the Lumbar Spine Provides Access to the Lumbar Spine With Few Early Complications. Clin Orthop 2016;474:2020-7. [Crossref] [PubMed]
- Kraiwattanapong C, Arnuntasupakul V, Kantawan R, et al. Malposition of Cage in Minimally Invasive Oblique Lumbar Interbody Fusion. Case Rep Orthop 2018;2018:9142074. [Crossref] [PubMed]
- Chang J, Kim JS, Jo H. Ventral Dural Injury After Oblique Lumbar Interbody Fusion. World Neurosurg 2017;98:881.e1-881.e4. [Crossref] [PubMed]
- Clark AJ, Safaee MM, Khan NR, et al. Tubular microdiscectomy: techniques, complication avoidance, and review of the literature. Neurosurg Focus 2017;43:E7. [Crossref] [PubMed]
- Zhou Y, Luo G, Chu T, et al. The biomechanical change of lumbar unilateral graded facetectomy and strategies of its microsurgical reconstruction: report of 23 cases. Zhonghua Yi Xue Za Zhi 2007;87:1334-8. [PubMed]
- Mody MG, Nourbakhsh A, Stahl DL, et al. The prevalence of wrong level surgery among spine surgeons. Spine 2008;33:194-8. [Crossref] [PubMed]
- Groff MW, Heller JE, Potts EA, et al. A survey-based study of wrong-level lumbar spine surgery: the scope of the problem and current practices in place to help avoid these errors. World Neurosurg 2013;79:585-92. [Crossref] [PubMed]
- Chin KR, Seale J, Cumming V. Avoidance of Wrong-level Thoracic Spine Surgery Using Sterile Spinal Needles: A Technical Report. Clin Spine Surg 2017;30:E54-8. [Crossref] [PubMed]
- Reitman CA. Pearls: Wrong-level Surgery Prevention. Clin Orthop 2016;474:636-9. [Crossref] [PubMed]
- Hsiang J. Wrong-level surgery: A unique problem in spine surgery. Surg Neurol Int 2011;2:47. [PubMed]
- Malham GM, Goss B, Blecher C. Percutaneous Pedicle Screw Accuracy with Dynamic Electromyography: The Early Experience of a Traditionally Open Spine Surgeon. J Neurol Surg A Cent Eur Neurosurg 2015;76:303-8. [Crossref] [PubMed]
- Li YB, Wang XD, Yan HW, et al. The Long-term Clinical Effect of Minimal-Invasive TLIF Technique in 1-Segment Lumbar Disease. Clin Spine Surg 2017;30:E713-9. [Crossref] [PubMed]
- Lee WC, Park JY, Kim KH, et al. Minimally Invasive Transforaminal Lumbar Interbody Fusion in Multilevel: Comparison with Conventional Transforaminal Interbody Fusion. World Neurosurg 2016;85:236-43. [Crossref] [PubMed]
- Watkins RG, Hanna R, Chang D, et al. Sagittal alignment after lumbar interbody fusion: comparing anterior, lateral, and transforaminal approaches. J Spinal Disord Tech 2014;27:253-6. [Crossref] [PubMed]
- Lim JK, Kim SM. Radiographic Results of Minimally Invasive (MIS) Lumbar Interbody Fusion (LIF) Compared with Conventional Lumbar Interbody Fusion. Korean J Spine 2013;10:65-71. [Crossref] [PubMed]
- Bae HJ, Cho TG, Kim CH, et al. Aortic Injury during Transforaminal Lumbar Interbody Fusion. Korean J Spine 2017;14:118-20. [Crossref] [PubMed]


