Acute post-operative airway complications following anterior cervical spine surgery and the role for cricothyrotomy
Introduction
When pain and disability from cervical spondylosis and disc degeneration become refractory to conservative treatment, surgical interventions such as anterior cervical discectomy and fusion (ACDF) or anterior cervical corpectomy and fusion (ACCF) may be necessary to treat or prevent cervical myelopathy or radiculopathy. Surgical treatment relieves symptoms by decompressing the spinal cord and nerve roots. ACDF is a common procedure and most patients have excellent results (1-3). A long list of complications may arise after anterior cervical spine surgery (ACSS), with dysphagia being the most common (4-11). Post-operative adverse events related to the airway occur in up to 14% of patients after multi-level ACSS with or without fusion (7,12,13). Retropharyngeal edema occurs in up to 6% of patients with in the greatest risk arising in multilevel surgery (14,15). A post-operative hematoma occurs in up to 2.4% in ACSS (4,5,10,11,13,15-19). Of patients undergoing single level ACDF, 2% may require reintubation (4,7,19-26) and as many as 5.2% may require reintubation after multilevel surgery (21,27). These are uncommon complications. However, airway obstruction is a potentially life-threatening event for which one must be prepared with an effective management plan. Symptoms of airway compromise after ACSS requires immediate attention, as the situation may quickly proceed to respiratory arrest. Obtaining emergent control of the airway is the key to survival of such patients. Knowing how to perform emergency intubation, cricothyrotomy or tracheostomy without delay is critical. Unfortunately, these challenging situations may occur during off-hours when available support is limited. The objective of this review is to describe the different etiologies of post-operative airway compromise following ACSS, the predictable timeline in which they occur, and the most appropriate treatment and management for each. We place special emphasis on the timing for establishing an emergent airway, and the proper surgical technique for a cricothyrotomy, a lifesaving procedure that is not commonly performed by most physicians.
Clinical anatomy and surgical approach
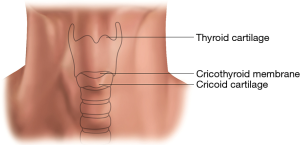
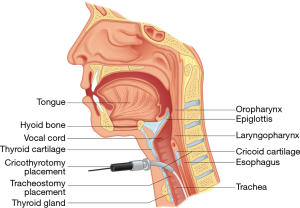
Effective treatment for a patient with airway compromise following ACSS requires knowledge of the neck and upper respiratory tract anatomy as well as procedural skills. The neck contains many vital structures within a small space. Important superficial landmarks include the cricoid and thyroid cartilages (Figure 1). Palpating from the inferior to superior, the cricoid cartilage is the first prominence and the thyroid cartilage is the second. Typically, in males the thyroid cartilage (“Adam’s apple”) is more prominent. In women the cricoid cartilage is usually more pronounced. The soft spot between these two landmarks is the cricothyroid membrane, which is incised during a cricothyrotomy.
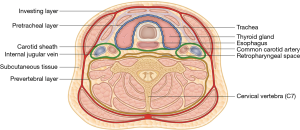
Internally, the neck is divided into three deep fascial layers (Figure 2). The first or investing layer is most superficial. The second or pretracheal (visceral) layer encloses the trachea, esophagus and thyroid gland. The third or prevertebral layer surrounds the cervical vertebral column. The carotid sheath is formed by contributions from all three fascial layers and encompasses the common carotid and internal carotid arteries, the internal jugular vein, the lymphatic plexus and cranial nerves IX–XII. The retropharyngeal space is located between the pretracheal and prevertebral fascial layers and is a potential space for hematomas following ACSS (15,16). The thyroid gland overlies the trachea and cricoid cartilage (Figure 3). It is supplied by the superior and inferior thyroid arteries. Injury to the superior thyroid artery may lead to a post-operative hematoma (16,28). The thyroid cartilage is located at C4, just superior to the thyroid gland, while the cricoid cartilage is located at C6. These structures are at increased risk of injury during the anterior approach in ACSS.
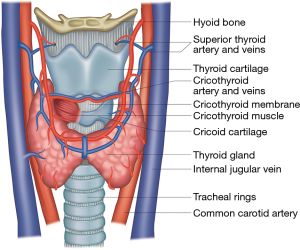
The surgical technique for ACDF was first described by Robinson and Smith, and has become the standard approach to most ACSS (1,2,29,30). With the patient supine on the operating room (OR) table, either a transverse or longitudinal incision can be made to expose the cervical vertebrae depending on the numbers of vertebral levels necessary. For C4–5, the incision is made at the level of the thyroid cartilage while for C5–7, the incision is made at the level of the cricoid cartilage. After splitting the platysma and deep cervical fascia, blunt dissection is made medially to the carotid sheath and down through the prevertebral fascia. The trachea and larynx must be gently retracted medially. Great care should be taken to limit excessive retraction to prevent possible development of edema. During closure, good hemostasis should be achieved to prevent hematoma formation.
Clinical presentation
Patients developing respiratory distress after ACSS may present in various ways. They may progress from being asymptomatic to having partial or complete airway obstruction within a few minutes or gradually over several days. Early on, they may complain of difficulty breathing, swallowing, and talking, exacerbated in the supine position (16). Patients may complain they are suffocating or that the cervical collar is becoming too tight (31). They may exhibit voice changes (14,16). However, oxygen saturation will often remain normal. Therefore, a satisfactory measurement with pulse oximetry may be misleading and delay intervention. The carbon dioxide tension measured in an arterial blood gas provides a better objective assessment of the patient’s respiratory status (21). The patient may become restless and agitated as carbon dioxide levels increase; hypoxemia may or may not be present (14,16). They may have a look of fear or panic associated with dyspnea (16). In later stages, the patient may use accessory respiratory muscles, or demonstrate inspiratory stridor or cyanosis (16).
Various etiologies of acute postoperative airway compromise following ACSS present in a predictable timeline (Figure 4), with most adverse airway events occurring 24–48 hours after surgery (7,17,32,33). Severe cases will require reintubation. Nagoshi et al. performed a retrospective multi-center cohort study of 8,887 ACSS patients and found that 9 patients required reintubation, with 6 patients developing airway compromise within 24 hours of surgery and the other 3 patients between 5–7 days (24). Patients should be counseled on the symptoms and advised to seek immediate care as delay can result in fatality.
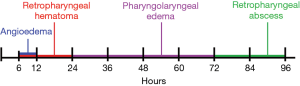
Angioedema
Although uncommon, angioedema may cause respiratory distress in the immediate post-operative setting, such as in the post-anesthesia care unit (34). Patients can develop difficulty breathing with neck swelling extending into the face and oropharynx (34). They may also have a massively swollen tongue protruding from their mouth (34). It is essential to differentiate angioedema from a retropharyngeal hematoma to provide proper treatment. Their early findings can be very similar, but reopening the surgical wound to evacuate a hematoma will not address the underlying cause nor relieve the symptoms of angioedema (34). Most cases of angioedema are idiopathic, and these cases are treated with intravenous (IV) corticosteroids, antihistamines, and/or subcutaneous (or IV) epinephrine (34). Hereditary angioedema represents 1–6% of cases (34), and does not respond to corticosteroids or antihistamines (35). Instead, IV plasma-derived C1 esterase inhibitor (C1-INH) can be given prophylactically or as a lifesaving treatment. In all cases of angioedema, there should be a lower threshold for intubation as these patients can rapidly progress to respiratory failure. Endotracheal intubation may quickly become difficult due to progressive swelling and disruption of the normal cervical anatomy.
Retropharyngeal hematoma
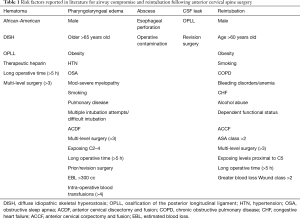
Clinically significant hematomas typically appear within 6–12 hours of surgery (5,14,16). Lied et al. found all wound hematomas presented within 6 hours after surgery (5). Song found 67% of retropharyngeal hematomas occur within 24 hours and the remaining 33% within 72 hours (13). O’Neill et al. found 65% of retropharyngeal hematomas occur within 24 hours and the remaining 35% within 6 days (15). Patients who develop a retropharyngeal hematoma typically form a tense mass under the incision, swelling of the anterior neck, and possible tracheal deviation (16,28,33,36-39). Multiple studies have investigated risk factors for post-operative retropharyngeal hematoma after cervical spine surgery (Table 1) (13,15,16,40). Other factors associated with hematoma formation include coagulopathies, excessive intra-operative blood loss, excessive postoperative hypertension, and prolonged excessive coughing during extubation (16). Inadequate intra-operative hemostasis is also a risk factor that can lead to post-operative hematomas. However, hematomas can still form despite apparently adequate intraoperative hemostasis (11,13,15,16,38). At the time of diagnosis, a retropharyngeal hematoma should be immediately evacuated through the existing surgical incision to prevent further progression of respiratory distress. O’Neill et al. found that of the 17 patients who developed a wound hematoma following ACSS, 2 required cricothyrotomy (15).
Full table
Pharyngolaryngeal edema
If signs and symptoms of respiratory distress develop between 12 to 72 hours post-operatively, pharyngolaryngeal edema should be highly suspected as the cause of respiratory failure (7,14). Patients can have various clinical presentations. They may progress slowly with difficulty talking and subtle changes in voice quality. Dyspnea may be exacerbated in supine position. Some patients may suddenly collapse with respiratory arrest. Multiple authors have attempted to define the risk factors leading to this complication (Table 1) (7,10,12,21,23,25-27,41-43). Patients with multiple risk factors should have a very low threshold for maintaining post-operative intubation for 24–48 hours and transferring to intensive critical care unit for observation. Patients with pharyngolaryngeal edema may pose a significant challenge for reintubation, and potential emergency cricothyrotomy should always be considered.
Cervical abscess
Cervical abscess typically takes days to weeks to develop, and is the most likely cause of airway compromise more than 72 hours after surgery (14). A patient with a cervical abscess may complain of difficulty swallowing or choking sensation especially at night (44). They may also notice neck swelling, fevers, and worsening leukocytosis (45). Treatment should include irrigation and debridement as well as appropriate IV antibiotics. Specifically, a retropharyngeal abscess may develop as a result of esophageal perforation or operative contamination (Table 1) (45).
Cerebrospinal fluid (CSF) leak
An intraoperative dural tear can lead to a CSF leak. This is a rare complication, which even less frequently leads to respiratory distress. CSF leaks are usually detected in the OR. However, if the tear is missed, it can present within a variable timeframe with similar findings to a hematoma, such as swelling of the anterior neck, formation of a tense mass, and possible tracheal deviation (16,28,33,36-39). Greater risk for dural tears is associated with more complex surgeries including revisions and patients with severe ossification of the posterior longitudinal ligament (OPLL) (Table 1) (4,36). When a dural tear is observed intraoperatively it must be immediately repaired, often followed by insertion of a closed lumbar CSF drain (6,11,36). If a CSF leak is not treated appropriately, recurrences may prompt multiple reintubations (46).
Imaging
Imaging is a routine part of pre- and post-operative evaluation of the cervical spine surgical patient. Plain radiographs are the mainstay of post-operative evaluation. However, computed tomography (CT) is superior for evaluation of post-operative complications, particularly soft-tissue abnormalities that could cause airway compromise. MRI is more sensitive than CT in detecting soft tissue pathology around the cervical spine (47). However, MRI also has several disadvantages, such as increased artifacts, cost, and significantly longer acquisition time (47). Thus, MRI is generally impractical for emergent settings such as airway compromise.
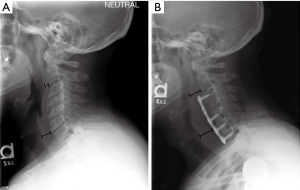
In cervical spine radiographs, the airway is best evaluated on the lateral view. Radiographic prevertebral soft tissue swelling (PSTS) is most commonly due to pharyngolaryngeal edema (33). Many studies have used similar methods for quantifying PSTS after ACSS, most commonly by measuring the distance between the anterior margin of the vertebral body to the posterior margin of the airway shadow at the same level (Figure 5) (22,32,48,49). PSTS should be identified by comparing a post-operative study to a pre-operative study rather than with absolute thickness, as there is a wide range of normal prevertebral soft tissue thicknesses (48). Most severe PSTS is typically found in the region of C4 (32). PSTS is greatest on post-operative days 2 and 3, followed by a gradual decrease after day 4 (22,49). However, patients most frequently experienced dyspnea on post-operative days 0 to 2, which differs from noted maximum soft tissue swelling on post-operative days 2 or 3 (49). Thus, degree of PSTS alone does not necessarily predict risk of dyspnea, and other contributing factors such as hematoma must also be considered.
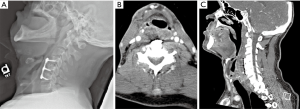
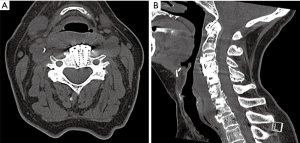
Other common causes of postoperative airway compromise include hematoma, seroma, and abscess. Hematoma and seroma typically develop within the prevertebral soft tissue. On radiographs, these entities appear similar to each other and to PSTS. CT of the neck with IV contrast is useful for evaluating soft tissue swelling. Infected fluid collections or abscesses will have peripheral enhancement with IV contrast (Figure 6) (44), whereas hematomas will typically not enhance with IV contrast, and seromas may or may not have thin peripheral enhancement. A seroma will have uniform, simple-fluid density on CT, whereas an acute hematoma usually appears denser and more heterogeneous (Figure 7). CSF leaks, while rare, can lead to formation of a pseudomeningocele that can cause delayed airway compromise (46). They present on CTs as a low-density fluid collection with thin peripheral enhancement (50).
Perioperative corticosteroids
Corticosteroids have been used in the past in the hope of decreasing pharyngolaryngeal edema following prolonged tracheal intubation, and surgeries such as thyroidectomy and carotid endarterectomy (51,52). For patients undergoing ACSS, the perioperative administration of IV corticosteroids remains controversial. Numerous studies have been conducted using various protocols, and have found a reduction in PSTS on lateral radiographs or on flexible endoscopic airway examination (31,32,53,54). However, other studies have found no significant difference in extubation timing, airway compromise, or reintubation rate following intra-operative corticosteroid treatment (20,51,55). While corticosteroids are known to cause nonunion in bone healing, several studies have found no significant difference in bony fusion rates at 12 months (20,55). Other side effects of systemic corticosteroids include hyperglycemia, infection, gastritis, peptic ulcer disease, deep vein thrombosis (DVT), osteoporosis and avascular necrosis. Local administration of retropharyngeal steroids has the advantage of a lower risk of systemic complications (32). Thus, the surgical team should weigh the risks and benefits of perioperative steroids for each patient to provide the best clinical outcome.
Postoperative intubation protocol
There are multiple algorithms established by different national and international specialty societies as a guide for “difficult” airway placements in patients that require anesthesia. Unfortunately, there is no established standard algorithm either for postoperative airway management after ACSS or for how best to secure the airway should reintubation be required (16,41). We believe that it is helpful to preemptively identify patients at increased risk for postoperative airway compromise and need for reintubation. Recognizing these concerns, Epstein et al. developed a conservative protocol with for patients undergoing multi-level anterior-posterior spine surgery (26). All patients remained intubated overnight after surgery and were extubated only after direct fiberoptic assessment. While most patients (40/58) could be extubated on post-operative day 1, a substantial number (31%) had delayed extubation. Only one (1.7%) patient required emergent reintubation.
Palumbo et al. suggested an airway protection protocol following ACSS based on surgical risk factors (7), stratifying the patients into low-, intermediate-, and high-risk categories (14). Patients in the low-risk group may be safely extubated after surgery in the OR. Patients in the high-risk group had multiple surgical risks factors, were routinely ventilated overnight, and underwent delayed extubation (14). Patients in the intermediate-risk group had only one surgical risk factor. Palumbo et al. recommended evaluating the secondary risk factors to determine if the patient should be left intubated for overnight observation.
Kim et al. also created an airway management protocol based on risk factors (41). The patients would remain intubated overnight if they had any one of five risk factors including: (I) exposure of greater than 3 cervical levels; (II) operative site on C3–4 or above; (III) greater than 5 hours operative time; (IV) blood loss greater than 300 mL; (V) a significant medical comorbidity (7,41). In addition, the cuff-leak test and lateral radiographs were repeated at 12 hours intervals until the patients were extubated. The protocol group had a significantly reduced postoperative airway complication rate than the non-protocol group (41).
Patients left intubated after surgery should have the head of the bed elevated to 30 degrees to improve ventilation and venous drainage. Patients should be extubated only when appropriate physicians and supporting staff are available to manage a failed extubation. If a patient develops laryngeal edema and cannot be reintubated, either transtracheal jet ventilation or an emergent cricothyrotomy should be performed (16). If there is complete airway obstruction, jet ventilation is precluded given the inability for exhaled gas to exit. Unfortunately, after ACSS, postoperative swelling and anatomical distortion may result in increased rates of failed intubations (13). Timely restoration of ventilation and oxygenation is critical to avoid asphyxia-induced cardiac arrest and brain injury. Therefore, an emergency cricothyrotomy kit should be readily accessible near the patient’s bedside, including on the floor unit. Of note, cricothyrotomy is contraindicated in patients who are under 12 years of age.
Sequence of resuscitation after respiratory arrest
The sequence of resuscitation efforts is typically based on the assumption that after cardiac arrest, the brain can recover if adequate oxygen delivery resumes within 5 minutes (56). However, this time limit was determined based on animal studies of cardiac arrest without prior asphyxia. Unfortunately, animal studies comparing cardiac arrest with or without prior asphyxia uniformly demonstrate that prior asphyxia leads to worse outcomes and reduces the potential time of cardiac arrest after which meaningful survival can be anticipated (57,58). We can assume that in patients who experience cardiac arrest after ACSS as a consequence of airway edema and obstruction, the cause of cardiac arrest is progressive hypoxemia and hypercarbia. Similarly, we strongly suspect that without immediate restoration of adequate oxygen delivery, such patients will likely be associated with catastrophic brain injury following cardiac arrest (59-61).
Like all procedures, experience is a critical factor for an efficient performance of emergent cricothyrotomy. Petrosoniak et al. noted that 20 emergency medicine residents, in a simulation lab, had a cricothyrotomy performance time of approximately 160 s (±48 s) including assessment to technical completion of the task prior to simulation training (62). Subsequent simulation training reduced this time to 100 s (±26 s). Many of these residents had witnessed or performed a cricothyrotomy simulation in the past, but only one resident had actually performed a cricothyrotomy on a patient. An observational study reported that experienced physicians needed a median of 73 s (range, 53–255 s), while inexperienced physicians required a median of 180 s to complete a cricothyrotomy using the standard technique in unfixed cadavers (63). An observational study of 44 paramedic students found that an average of 46 s (range, 29–63 s) were needed to complete a surgical cricothyrotomy (64). A performance time study would be beneficial using orthopedic and neurosurgery residents. Based on this data and assuming minimal experience with cricothyrotomy, we make the following recommendations regarding emergency airway management (which can be modified based on the operator’s level of experience):
- Immediately call for help so that another physician can oversee cardiac arrest management, such as chest compressions, pharmaceutical treatments, and defibrillation. We suggest adhering to the American Heart Association/American College of Cardiology Advanced Cardiovascular Life Support (ACLS) algorithm or the relevant algorithms in other countries (e.g., Resuscitation Council in the United Kingdom).
- Immediately upon arrival, ventilate a patient experiencing respiratory arrest with oxygen using a bag-valve mask system. Observe chest rise and level of oxygenation to assess the effectiveness of ventilation. An assistant can attach a pulse oximeter to the patient and a capnograph in line with the mask. Give particular attention to appropriate mask seal and position of mandible. The mask should have a circumferential seal over the perinasal and perioral face. The mandible should be advanced anteriorly to dilate the posterior oropharyngeal orifice.
- If ventilation is not effective within 1 minute, make 1 attempt at oral intubation using (preferably) video laryngoscopy or a conventional laryngoscope.
- If orotracheal intubation is not successful or if more than 2 minutes have passed since onset of respiratory arrest, proceed directly to cricothyrotomy.
Cricothyrotomy kit
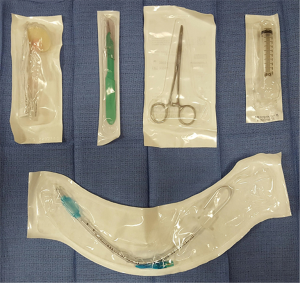
The emergency cricothyrotomy kit should only contain the essential instruments. The kit should include an antiseptic skin cleaning preparation such as ChloraPrepTM, 11- or 15-blade scalpel, hemostat, a small cuffed endotracheal or tracheostomy tube, and a 10-mL syringe (Figure 8). Kits are best kept on the hospital floor, in the resident room, and at the bedside of high-risk patients where they are readily accessible. Like code carts, all clinical staff should be aware of the location of emergency cricothyrotomy kits.
Steps for cricothyrotomy procedure
- Confirm that ventilation with bag valve mask and endotracheal intubation have failed;
- Position patient supine with the neck extended, exposing the anterior trachea;
- Clean the skin with antiseptic solution, such as a ChloraPrepTM. Double glove yourself;
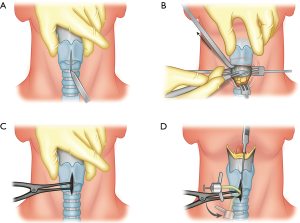
- Palpate laryngeal landmarks: thyroid cartilage notch, cricoid cartilage, and cricothyroid membrane (Figure 9A);
- Make a longitudinal midline skin incision from the thyroid notch to the cricoid cartilage and deep to the cricothyroid membrane (Figure 9A);
- Use blunt dissection to evacuate any hematomas making sure that the posterior aspect of the trachea is cleared. Having a suctioning device available may aid in exposure;
- Make a transverse incision through the cricothyroid membrane (Figure 9B). If the provider is unsure of the location of the cricothyroid membrane, a needle attached to a saline filled syringe can be inserted through the site. If there is return of air bubbles on aspiration, this will confirm location of the needle tip within the airway;
- Insert the hemostat and dilate the opening (Figure 9C);
- Insert the cuffed endotracheal tube or tracheostomy tube into the opening (Figure 9D);
- Inflate the cuff to prevent an air leak;
- Confirm endotracheal placement with return of end-tidal CO2, increase in O2 saturation, and chest rise;
- Do not let go of the tracheal tube until it is secured with tape;
- Take patient straight to the OR for formal wound exploration and conversion to a definitive tracheostomy (Figure 10).
Pearls and pitfalls
- Proper care of the at-risk airway begins prior to the need for a surgical airway. Patients at risk for airway compromise should be continuously monitored with pulse oximetry in an intermediate or stepdown unit with adequate nursing care. Communication between the surgical team and nursing staff regarding airway management should be part of the patient transfer process after surgery.
- Any changes in respiratory status should be immediately assessed. This may include bedside laryngoscopy to evaluate the airway. In the non-responsive patient with developing respiratory distress, cricothyrotomy should not be delayed by multiple intubation attempts. Delayed placement of a surgical airway is the most common reason for adverse outcomes in cases of airway obstruction.
- Airway management solutions should be initiated in parallel sequence. For example, calling for the materials for intubation should also include having the materials available to rapidly establish a surgical airway if needed.
- The patient’s neck should be positioned in extension to best show relevant laryngeal landmarks, and this may be the difference between inadequate versus good exposure of the cricothyroid membrane. Neck extension may be accomplished by placing a rolled blanket, towel, or sheet under the shoulders, and removing the pillow.
- Once the patient is adequately positioned, the surgeon should firmly grasp the larynx between their thumb and third/fourth finger of their non-dominant hand using their second finger to palpate the cricothyroid membrane. Pressure should be applied downward (posteriorly) bilaterally to retract the vertical skin incision as it is made and facilitate exposure of the cricothyroid membrane.
- The tracheostomy or endotracheal tube should be rotated from 90 degrees to the trachea to straight during insertion. If you are using an endotracheal tube with a flexible tip, the tip may flip superiorly instead of inferiorly toward the airway. Pointing the tip down and not inserting perpendicular to the skin will help direct the tip inferiorly. If the ETT is placed superiorly, it will become evident with oxygenation failing to improve and absence of return of end-tidal CO2 on monitoring. The tube should be removed and carefully reinserted. A rigid bougie, intubating stylet or flexible fiberoptic bronchoscope may aid with insertion.
- A cuffed, flexible tracheostomy tube such as a Shiley #4 or #6 or an endotracheal tube may be utilized. If difficultly is encountered with placement of a tracheostomy tube, the provider should switch to an endotracheal tube.
- Bleeding during an emergent surgical airway is difficult to avoid as there may be several, relatively large anterior jugular veins in the area. Staying as close to midline as possible will decrease this risk.
Conclusions
ACSS, such as ACDF, is becoming more common as these procedures produce good outcomes with low risk of complications. Unfortunately, some perioperative complications may quickly become debilitating or fatal. The most common causes of airway compromise during recovery from ACSS are pharyngolaryngeal edema and hematoma. Certain risk factors can be used to help stratify patients into low-, intermediate- and high-risk groups for post-surgical airway compromise. Physicians must be prepared to diagnose and immediately treat these life-threatening complications to prevent cardiopulmonary arrest, anoxic brain injury, or death. If intubation cannot be accomplished immediately on the first attempt, physicians should not hesitate to obtain a secure, surgical airway. Although patients have had meaningful recoveries after uncomplicated cardiac arrests in which successful resuscitation required greater than 5 minutes, patients experiencing cardiac arrest as a complication of asphyxia differ. A brain subjected to increasing degrees of hypercarbia and hypoxemia prior to cardiac arrest has no tolerance for delayed resuscitation (59). We recommend that an audit be performed on inpatient floors to ensure supplies for emergency surgical airways are readily available. We also recommend that all residents participate in emergency airway placement simulations during orientation and review this article annually.
Acknowledgements
This work was supported by the Virginia Commonwealth University (VCU) Health, Department of Orthopedic Surgery—by providing funding for figures.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rhee J, Voyadzis JM. Anterior Cervical Diskectomy and Fusion. In: Surgical Anatomy and Techniques to the Spine. 2014:131-8.
- Helgeson MD, Albert TJ. Anterior surgical approach to the cervical spine. In: Shen FH, Samartzis D, Fessler RG, editors. Textbook of the Cervical Spine. 1st edition. Philadelphia: Saunders Elsevier; 2014. p. 33-8.
- Wilson AS, Samartzis D, Shen FH. Anterior Cervical Diskectomy and Fusion. In: Textbook of the Cervical Spine. 2015:285-93.
- Daniels AH, Riew KD, Yoo JU, et al. Adverse events associated with anterior cervical spine surgery. J Am Acad Orthop Surg 2008;16:729-38. [Crossref] [PubMed]
- Lied B, Sundseth J, Helseth E. Immediate (0-6 h), early (6-72 h) and late (>72 h) complications after anterior cervical discectomy with fusion for cervical disc degeneration; discharge six hours after operation is feasible. Acta Neurochir 2008;150:111-8. [Crossref] [PubMed]
- Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy and fusion associated complications. Spine (Phila Pa 1976) 2007;32:2310-7. [Crossref] [PubMed]
- Sagi HC, Beutler W, Carroll E, et al. Airway Complications Associated With Surgery on the Anterior Cervical Spine. Spine (Phila Pa 1976) 2002;27:949-53. [Crossref] [PubMed]
- Macdonald RL, Fehlings MG, Tator CH, et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg 1997;86:990-7. [Crossref] [PubMed]
- Sinkiewicz A, Harat M, Furtak J. Complications after surgery of the anterior cervical spine. Neurol Neurochir Pol 1997;31:135-44. [PubMed]
- Boakye M, Patil CG, Ho C, et al. Cervical corpectomy: Complications and outcomes. Neurosurgery 2008;63:295-301; discussion 301-2. [PubMed]
- Tasiou A, Giannis T, Brotis AG, et al. Anterior cervical spine surgery-associated complications in a retrospective case-control study. J Spine Surg 2017;3:444-59. [Crossref] [PubMed]
- Kwon B, Yoo JU, Furey CG, et al. Risk factors for delayed extubation after single-stage, multi-level anterior cervical decompression and posterior fusion. J Spinal Disord Tech 2006;19:389-93. [Crossref] [PubMed]
- Song KJ, Choi BW, Lee DH, et al. Acute airway obstruction due to postoperative retropharyngeal hematoma after anterior cervical fusion: A retrospective analysis. J Orthop Surg Res 2017;12:19. [PubMed]
- Palumbo MA, Aidlen JP, Daniels AH, et al. Airway compromise due to laryngopharyngeal edema after anterior cervical spine surgery. J Clin Anesth 2013;25:66-72. [Crossref] [PubMed]
- O'Neill KR, Neuman B, Peters C, et al. Risk factors for postoperative retropharyngeal hematoma after anterior cervical spine surgery. Spine (Phila Pa 1976) 2014;39:E246-52. [Crossref] [PubMed]
- Palumbo MA, Aidlen JP, Daniels AH, et al. Airway Compromise Due to Wound Hematoma Following Anterior Cervical Spine Surgery. Open Orthop J 2012;6:108-13. [Crossref] [PubMed]
- Bertalanffy H, Eggert HR. Complications of anterior cervical discectomy without fusion in 450 consecutive patients. Acta Neurochir 1989;99:41-50. [Crossref] [PubMed]
- Viejo-Fuertes D, Liguoro D, Ansari M, et al. Complications following anterior approaches to the cervical spine. Review of 535 surgical procedures. Eur J Orthop Surg Traumatol 2000;10:177-81. [Crossref]
- Al Maaieh M, Taher F, Iyer S, et al. The Immediate Safety Profile after Complex Anterior Cervical Reconstruction Surgery. MOJ Orthop Rheumatol 2015;2:4-9. [Crossref]
- Jeyamohan SB, Kenning TJ, Petronis KA, et al. Effect of steroid use in anterior cervical discectomy and fusion: a randomized controlled trial. J Neurosurg Spine 2015;23:137-43. [Crossref] [PubMed]
- Li H, Huang Y, Shen B, et al. Multivariate analysis of airway obstruction and reintubation after anterior cervical surgery: A Retrospective Cohort Study of 774 patients. Int J Surg 2017;41:28-33. [Crossref] [PubMed]
- Suk KS, Kim KT, Lee SH, et al. Prevertebral soft tissue swelling after anterior cervical discectomy and fusion with plate fixation. Int Orthop 2006;30:290-4. [Crossref] [PubMed]
- Marquez-Lara A, Nandyala SV, Fineberg SJ, et al. Incidence, outcomes, and mortality of reintubation after anterior cervical fusion. Spine (Phila Pa 1976) 2014;39:134-9. [Crossref] [PubMed]
- Nagoshi N, Fehlings MG, Nakashima H, et al. Prevalence and Outcomes in Patients Undergoing Reintubation After Anterior Cervical Spine Surgery: Results From the AOSpine North America Multicenter Study on 8887 Patients. Global Spine J 2017;7:96S-102S. [Crossref] [PubMed]
- Lim S, Kesavabhotla K, Cybulski GR, et al. Predictors for Airway Complications Following Single- and Multilevel Anterior Cervical Discectomy and Fusion. Spine (Phila Pa 1976) 2017;42:379-84. [Crossref] [PubMed]
- Epstein NE, Hollingsworth R, Nardi D, et al. Can airway complications following multilevel anterior cervical surgery be avoided? J Neurosurg 2001;94:185-8. [PubMed]
- Emery SE, Smith MD, Bohlman HH. Upper-airway obstruction after multilevel cervical corpectomy for myelopathy. J Bone Joint Surg Am 1991;73:544-51. [Crossref] [PubMed]
- Yu NH, Jahng TA, Kim CH, et al. Life-threatening late hemorrhage due to superior thyroid artery dissection after anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2010;35:E739-42. [Crossref] [PubMed]
- Smith GW, Robinson RA. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am 1958;40-A:607-24. [Crossref] [PubMed]
- Jho HD. Decompression via microsurgical anterior foraminotomy for cervical spondylotic myelopathy. Technical note. J Neurosurg 1997;86:297-302. [Crossref] [PubMed]
- Pedram M, Castagnera L, Carat X, et al. Pharyngolaryngeal lesions in patients undergoing cervical spine surgery through the anterior approach: contribution of methylprednisolone. Eur Spine J 2003;12:84-90. [PubMed]
- Lee SH, Kim KT, Suk KS, et al. Effect of Retropharyngeal Steroid on Prevertebral Soft Tissue Swelling Following Anterior Cervical Discectomy and Fusion; A Prospective, Randomized Study. Spine 2011;36:2286-92. [Crossref] [PubMed]
- Sethi R, Tandon MS, Ganjoo P. Neck hematoma causing acute airway and hemodynamic compromise after anterior cervical spine surgery. J Neurosurg Anesthesiol 2008;20:69-70. [Crossref] [PubMed]
- Krnacik MJ, Heggeness MH. Severe angioedema causing airway obstruction after anterior cervical surgery. Spine (Phila Pa 1976) 1997;22:2188-90. [Crossref] [PubMed]
- Umerani MS, Alzahrani K, Mostafa GA. Hereditary angioedema: A rare presentation after anterior cervical discectomy and fusion. Asian J Neurosurg 2015;10:253-5. [Crossref] [PubMed]
- Chang HS, Kondo S, Mizuno J, et al. Airway obstruction caused by cerebrospinal fluid leakage after anterior cervical spine surgery. A report of two cases. J Bone Joint Surg Am 2004;86-A:370-2. [Crossref] [PubMed]
- Roy SP. Acute postoperative neck hematoma. Am J Emerg Med 1999;17:308-9. [Crossref] [PubMed]
- Quick E, Byard RW. Postoperative Cervical Soft Tissue Hemorrhage with Acute Upper Airway Obstruction. J Forensic Sci 2013;58:S264-6. [Crossref] [PubMed]
- Tang SJ, Rao RD. Perioperative and Approach-Related Complications Associated with Anterior Cervical Surgery. Semin Spine Surg 2009;21:148-55. [Crossref]
- Skolasky RL, Thorpe RJ Jr, Wegener ST, et al. Complications and mortality in cervical spine surgery: racial differences. Spine (Phila Pa 1976) 2014;39:1506-12. [Crossref] [PubMed]
- Kim M, Choi I, Park JH, et al. Airway Management Protocol After Anterior Cervical Spine Surgery: Analysis of the Results of Risk Factors Associated With Airway Complication. Spine (Phila Pa 1976) 2017;42:E1058-E1066. [Crossref] [PubMed]
- Basques BA, Hijji FY, Khechen B, et al. Sex Differences for Anterior Cervical Fusion: Complications and Length of Stay. Spine (Phila Pa 1976) 2018;43:1025-30. [PubMed]
- Buerba RA, Giles E, Webb ML, et al. Increased risk of complications after anterior cervical discectomy and fusion in the elderly: An analysis of 6253 patients in the American College of Surgeons National Surgical Quality Improvement Program database. Spine (Phila Pa 1976) 2014;39:2062-9. [Crossref] [PubMed]
- Gwinnutt CL, Walsh GR, Kumar R. Airway obstruction after anterior cervical spine surgery. J Neurosurg Anesthesiol 1992;4:199-202. [Crossref] [PubMed]
- Kuriloff DB, Blaugrund S, Ryan J, et al. Delayed neck infection following anterior spine surgery. Laryngoscope 1987;97:1094-8. [Crossref] [PubMed]
- Penberthy A, Roberts N. Recurrent acute upper airway obstruction after anterior cervical fusion. Anaesth Intensive Care 1998;26:305-7. [Crossref] [PubMed]
- Hayashi D, Roemer FW, Mian A, et al. Imaging features of postoperative complications after spinal surgery and instrumentation. AJR Am J Roentgenol 2012;199:W123-9. [Crossref] [PubMed]
- Chen MY, Bohrer SP. Radiographic measurement of prevertebral soft tissue thickness on lateral radiographs of the neck. Skeletal Radiol 1999;28:444-6. [Crossref] [PubMed]
- Song KJ, Choi BW, Kim HY, et al. Efficacy of postoperative radiograph for evaluating the prevertebral soft tissue swelling after anterior cervical discectomy and fusion. Clin Orthop Surg 2012;4:77-82. [Crossref] [PubMed]
- Karasick D, Vaccaro R, Schweitzer ME. Complications of Cervical Spine Fusion: Imaging Features. AJR Am J Roentgenol 1997;169:869-74. [Crossref] [PubMed]
- Emery SE, Akhavan S, Miller P, et al. Steroids and risk factors for airway compromise in multilevel cervical corpectomy patients: a prospective, randomized, double-blind study. Spine (Phila Pa 1976) 2009;34:229-32. [Crossref] [PubMed]
- François B, Bellissant E, Gissot V, et al. 12-H Pretreatment With Methylprednisolone Versus Placebo for Prevention of Postextubation Laryngeal Oedema: a Randomised Double-Blind Trial. Lancet 2007;369:1083-9. [Crossref] [PubMed]
- Weinstein J, Schroeder JE, Sama AA, et al. Effect of Steroid Placement on a Gelatin Sponge and Soft Tissue Swelling Following Anterior Cervical Discectomy and Fusion: A Radiological Analysis. Spine J 2014;14:S116. [Crossref]
- Song KJ, Lee SK, Ko JH, et al. The clinical efficacy of short-term steroid treatment in multilevel anterior cervical arthrodesis. Spine J 2014;14:2954-8. [Crossref] [PubMed]
- Zadegan SA, Jazayeri SB, Abedi A, et al. Corticosteroid Administration to Prevent Complications of Anterior Cervical Spine Fusion: A Systematic Review. Global Spine J 2018;8:286-302. [Crossref] [PubMed]
- Weisfeldt ML, Becker LB. Resuscitation after cardiac arrest: A 3-phase time-sensitive model. JAMA 2002;288:3035-8. [Crossref] [PubMed]
- Vaagenes P, Safar P, Moossy J, et al. Asphyxiation versus ventricular fibrillation cardiac arrest in dogs. Differences in cerebral resuscitation effects - a preliminary study. Resuscitation 1997;35:41-52. [Crossref] [PubMed]
- Chen B, Chen G, Dai C, et al. Comparison of Quantitative Characteristics of Early Post-resuscitation EEG Between Asphyxial and Ventricular Fibrillation Cardiac Arrest in Rats. Neurocrit Care 2018;28:247-56. [Crossref] [PubMed]
- Varvarousis D, Varvarousi G, Iacovidou N, et al. The pathophysiologies of asphyxial vs dysrhythmic cardiac arrest: Implications for resuscitation and post-event management. Am J Emerg Med 2015;33:1297-304. [Crossref] [PubMed]
- Kolar M, Križmarić M, Klemen P, et al. Partial pressure of end-tidal carbon dioxide successful predicts cardiopulmonary resuscitation in the field: A prospective observational study. Crit Care 2008;12:R115. [Crossref] [PubMed]
- Eastwood GM, Young PJ, Bellomo R. The impact of oxygen and carbon dioxide management on outcome after cardiac arrest. Curr Opin Crit Care 2014;20:266-72. [Crossref] [PubMed]
- Petrosoniak A, Ryzynski A, Lebovic G, et al. Cricothyroidotomy In Situ Simulation Curriculum (CRIC Study): Training Residents for Rare Procedures. Simul Healthc 2017;12:76-82. [Crossref] [PubMed]
- Mutzbauer TS, Munz R, Helm M, et al. Emergency cricothyrotomy - puncture or anatomical preparation? Peculiarities of two methods for emergency airway access demonstrated in a cadaver model. Anaesthesist 2003;52:304-10. [Crossref] [PubMed]
- Johnson DR, Dunlap A, McFeeley P, et al. Cricothyrotomy performed by prehospital personnel: A comparison of two techniques in a human cadaver model. Am J Emerg Med 1993;11:207-9. [Crossref] [PubMed]