
Outcomes of direct lateral interbody fusion (DLIF) in an Australian cohort
Introduction
For patients with back pain with or without leg pain refractory to non-surgical care, spinal fusion is an alternative option for carefully selected patients for a variety of conditions including degenerative disc disease with or without radiculopathy, instability, adjacent segment disease, spondylolisthesis and stenosis (1). Traditionally, fusion via an open approach allowed for direct access to posterior spinal and paraspinal structures, permitting effective decompression and stabilization over the fused segments (2,3). However, in recent years, minimally invasive vertebral interbody fusion has gained popularity. Potential benefits of this approach include: reduced surgical trauma, improved morbidity rates, reduced blood loss and shortened hospital stay (4-8).
Direct lateral interbody fusion (DLIF) is a recently introduced, minimally invasive technique where a lateral approach to the intervertebral space is utilized for the placement of a large intervertebral fusion cage. In comparison to more conventional approaches, including anterior lumbar interbody fusion (ALIF) and posterior lumbar interbody fusion (PLIF), DLIF, by accessing the spine via a small cutaneous incision and muscle-splitting transpsoas retroperitoneal approach, does not compromise the anterior and posterior longitudinal ligaments, nor does it disrupt the posterior spinal musculature (9). Furthermore, this approach mitigates many of the vascular and visceral risks associated with ALIF in addition to minimising the neural complications, muscular trauma and bony resections associated with PLIF (10-16). Accordingly, DLIF aims to minimize the postoperative morbidity associated with open fusion and other minimally invasive techniques (9). This has proved particularly useful in elderly patients requiring correction of degenerative scoliosis (9,17).
To date, DLIF is reported to be associated with little tissue trauma, minimal blood loss, low reported postoperative pain, short hospital stays and a fast return to activities of daily living (18-23). Consequently, since its introduction in 2006, DLIF has gained popularity. However, due to reduced anatomical exposure and potentially decreased visualization, there is growing concern pertaining to potential complications specific to the DLIF approach, including psoas muscle injury and lumbar nerve plexus injuries resulting in hip flexor weakness and anterior thigh pain, numbness and/or dysesthesias (19,22,24-27). This is further complicated by the variability in lumbar plexus anatomy, which at times complicates identification of a safe suitable working corridor under fluoroscopy (28,29).
Presently there is a relative paucity of DLIF studies on patients with spinal disease, particularly in an Australian population, and thus, further reports on clinical and radiological outcomes and complications are necessary to validate this surgical approach. As such, the purpose of this study was to assess the clinical and radiological outcomes and the complication rates in the first 50 consecutive patients to have undergone the DLIF approach by a single neurosurgeon in Sydney, Australia.
Methods
Patient population
A prospective analysis of the first 50 consecutive patients to undergo DLIF by a single neurosurgeon between 2010 and 2014 was performed. Institutional human ethics approval was obtained from the Western Sydney Local Health District Human Research Ethics Committee prior to commencement of the review (LNR/14/WMEAD/425).
The inclusion criteria included those patients aged greater than 18 years with spinal disease at vertebral levels T11–L5 with symptoms not responding to conservative management for at least 6 months and where the senior author felt that DLIF was indicated. The surgical indications included radiculopathy, adjacent segment disease, spondylosis, spondylolisthesis, spinal stenosis and degenerative scoliosis. Exclusion criteria included patients with degenerative disease involving the L5/S1 level or L4/5 level in patients with a high iliac crest due to associated restriction of access to the disc space. Furthermore, patients with osteoporosis (T-score ≤−2.5), who were pregnant, had discitis, or who were unfit for general anaesthesia were excluded.
Surgical technique
Patients were positioned in the lateral decubitus position under general anaesthesia. Image intensification (II) was used to confirm the appropriate spinal level, the site of skin incision was marked and the surgical field was sterilized and draped. The DLIF technique involved a standard muscle-splitting retroperitoneal approach using blunt dissection. A transpsoas K wire was inserted into the anterolateral disc space under II guidance and a series of muscle dilators were utilized before placement of a 3 bladed retractor to expose the lateral annulus. Triggered and free running electromyography (EMG) monitoring was used to avoid injury to the lumbar plexus and ensure neural elements were not across the operative field. After lateral annulotomy, a thorough discectomy and endplate preparation using cup and box curettes was performed. The contralateral annulus was then released and the disc space assessed with trial implants before inserting an appropriate sized polyether ether ketone (PEEK) interbody implant filled with either Tricalcium Phosphate Vitoss® combined with Infuse® (DLIFs performed prior to August 2013) or allograft Crunch® combined with Infuse®. Patients under 80 years of age were then augmented with posterior percutaneous pedicle screw fixation.
Clinical outcomes
Clinical outcomes, including Visual Analogue Scale (VAS) back pain scores, Oswestry Disability Index (ODI) and Roland Morris Disability Questionnaire (RMDQ) surveys were obtained pre- and postoperatively.
Radiological evaluation
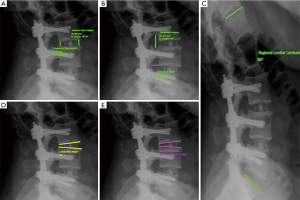
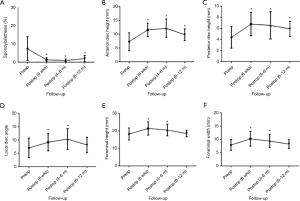
Radiological outcomes were assessed pre- and postoperatively using a combination of magnetic resonance imaging scans, computed tomography scans and plain erect X-rays of the lumbosacral spine. The outcomes that were measured included spondylolisthesis, anterior and posterior disc height, local disc angle, lumbar lordosis and foraminal height and width at the preoperative, 6 weeks, 6 months and 12 months postoperative follow-up stages (Figure 1). Measurements were performed using Surgimap Spine software (Nemaris, New York, NY, USA) and reviewed by an independent neurosurgeon. An independent radiologist assessed fusion rates at 6 and 12 months postoperatively.
Surgical complication assessment
All surgical complications were evaluated using operative notes, discharge summaries and postoperative clinical notes.
Statistical analysis
SPSS software (version 22.0; IBM Corporation, Armonk, NY, USA) was employed for all statistical analyses. In addition to descriptive statistics such as means, standard deviations, percentages, medians and interquartile ranges, differences between follow-up outcomes and preoperative measurements were compared using paired student’s t-tests. Two-tailed probabilities of less than 0.05 were considered significant.
Results
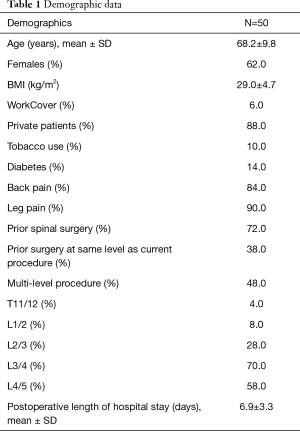
A total of 50 patients (84 levels) underwent DLIF for a number of indications, including radiculopathy (52%), adjacent segment disease (18%), spondylosis (10%), spondylolithesis (8%), spinal stenosis (4%) and degenerative scoliosis (8%). Ninety percent of the patients had experienced pain for greater than one year with 84% of patients having presented with back pain whilst 90.0% of patients presented with leg pain. Patient characteristics are summarised in Table 1. The mean patient age was 68.2±9.8 years and 62.0% were female. The mean body mass index (BMI) was 29.0±4.7. Baseline comorbidities included tobacco use (10.0%), diabetes mellitus (14.0%) and prior spinal surgery (72.0%). Of the patients who had prior spinal surgery, 38.0% of these patients had prior surgery at the same level as their current procedure. Forty-eight percent of patients had a multi-level procedure, with 32% of patients having a 2-level, 12% of patients having a 3-level and 4% having a 4-level DLIF. DLIF was performed at T11/12 (4.0%), L1/2 (8.0%), L2/3 (28.0%), L3/4 (70.0%), and L4/5 (58.0%). The mean postoperative length of hospital stay was 6.9±3.3 days.
Full table
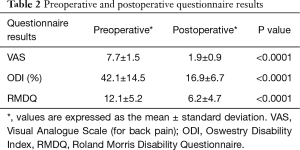
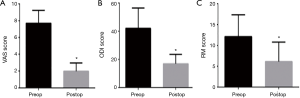
With respect to clinical outcome scores, the mean postoperative follow-up time was 2.1±1.2 years. The mean preoperative VAS pain score was 7.7±1.5 and improved to 1.9±0.9 postoperatively (P<0.0001). No patient had reported their postoperative pain to be the same or worse than their preoperative pain. The mean preoperative ODI was 42.1±14.5 and reduced to 16.9±6.7 postoperatively (P<0.0001). The mean preoperative RMDQ score was 12.1±5.2 and reduced to 6.2±4.7 postoperatively (P<0.0001). All clinical outcome scores significantly improved postoperatively (Table 2). Graphical representations of the clinical outcome scores are presented in Figure 2.
Full table
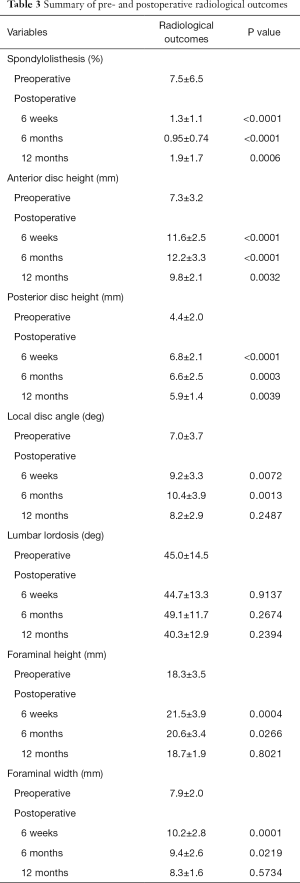
The mean preoperative spondylolisthesis was 7.5%±6.5%, which reduced to 1.3%±1.1% postoperatively at 6 weeks (P<0.0001), 0.95%±0.74% at 6 months (P<0.0001) and recurred to 1.9%±1.7% at 12 months (P=0.0006) follow-up but was still significant. All postoperative measurements were significantly reduced relative to preoperative spondylolisthesis values.
The mean preoperative anterior disc height was 7.3±3.2 mm. At 6 weeks postoperative follow-up, this was improved to 11.6±2.5 mm (P<0.0001). Measurements were 12.2±3.3 mm at 6 months (P<0.0001) and 9.8±2.1 mm at 12 months (P=0.0032) follow-up. In terms of posterior disc height, the mean preoperative measurement was 4.4±2.0 mm, compared to 6.8±2.1 mm at 6 weeks (P<0.0001), 6.6±2.5 mm at 6 months (P=0.0003), and 5.9±1.4 mm at 12 months (P=0.0039) follow-up. All postoperative measurements with respect to anterior and posterior disc height were significantly increased compared to preoperative measurements.
With respect to local disc angle, the mean preoperative angle was 7.0°±3.7°, compared to 9.2°±3.3° at 6 weeks (P=0.0072), 10.4°±3.9° at 6 months (P=0.0013) and 8.2°±2.9° at 12 months (P=0.2487) follow-up. Significant improvements were seen at 6 weeks and 6 months follow-up but no longer remained significant at 12 months follow-up.
In terms of lumbar lordosis, the preoperative mean was 45.0°±14.5°, compared to 44.7°±13.3° at 6 weeks (P=0.9137), 49.1°±11.7° at 6 months (P=0.2674) and 40.3°±12.9° at 12 months (P=0.2394) follow-up. There was no significant change in lumbar lordosis postoperatively compared to preoperatively.
The mean preoperative foraminal height measurements was 18.3±3.5 mm, compared to 21.5±3.9 mm at 6 weeks (P=0.0004), 20.6±3.4 mm at 6 months (P=0.0266), and 18.7±1.9 mm at 12 months (P=0.8021) follow-up. In terms of foraminal width, the preoperative mean was 7.9±2.0 mm, compared to 10.2±2.8 at 6 weeks (P=0.0001), 9.4±2.6 mm at 6 months (P=0.0219) and 8.3±1.6 mm at 12 months (P=0.5734) follow-up. Improvements in foraminal height and width were only significant at 6 weeks and 6 months postoperatively. Graphical representation and a summary of the radiological outcomes are presented in Figure 3 and Table 3, respectively.
Full table
The fusion rate following DLIF, as assessed by an independent radiologist, was 62.2% and 89.2% at 6 and 12 months, respectively.
A total of 6 patients (12%) had postoperative complications. Three patients (6%) had transient psoas muscle complications including pain-related hip flexion weakness [Medical Research Council (MRC) Grade 4] (n=1), psoas hematoma (n=1) and mild pain (n=1). These injuries had resolved entirely by 8 weeks postoperatively. Three patients (6%) had reported sensory neural complications including paresthesia in the distribution of the lateral cutaneous nerve of the thigh (n=2) and paresthesia in the distribution supplied by the femoral branch of the genitofemoral nerve (n=1). These complications had entirely resolved by 16 weeks postoperatively. None of the patients experienced deep vein thrombosis (DVT), infection, motor neural, dural, vascular or visceral injuries.
Discussion
In the context of an ageing Australian population, degenerative lumbar spine disease is increasing in prevalence and as such, the development and validation of effective surgical approaches to lumbar interbody fusion is paramount (30-33). DLIF offers numerous advantages over other minimally invasive spinal surgeries. By preserving the stabilizing elements of the spine (anterior longitudinal ligament, posterior longitudinal ligament, paraspinal muscles and facet joints), DLIF allows for conserved stability and anatomical load at the affected levels (34). Moreover, since there is no resection of the posterior bony elements, as with PLIF, iatrogenic instability is avoided. Compared to the posterior approach, the potential for paraspinal muscle and nerve root injuries and dural tears is reduced (35). In contrast to ALIF, DLIF minimizes the occurrence of peritoneal penetration and injuries to abdominal viscera whilst reducing the risk of vascular injury to the great vessels, including the abdominal aorta, inferior vena cava and iliolumbar vessels (10,11). Additionally, DLIF reduces risk of injury to the sympathetic chain (10,11).
Clinically, this study has demonstrated that DLIF significantly reduces back pain and improves functional outcomes in the long term. These findings are consistent with functional outcome scores in those studies looking at lateral interbody fusion for spinal deformity (9). Lee et al. noted a VAS score improvement of 66.7% and an ODI reduction from 39.9% to 11.1% in patients who underwent DLIF for degenerative lumbar disease (21). This is consistent with our finding of a 74.3% improvement in VAS score and an ODI reduction from 42.1% to 16.9% in patients with degenerative spinal disease. These clinical improvements are similar to those reported for more conventional approaches such as transforaminal lumbar interbody fusion (TLIF) and ALIF (36-40).
Consequent to its surgical approach, DLIF allows for both excellent disc space preparation and the use of large interbody implants. This results in increased contact between the cage and endplate, creating a biomechanically sound environment for bone healing and fusion (34,41). Furthermore, lateral fusion allows for restoration of disc height, correction of spinal alignment and through indirect decompression, effective decompression of the nerve roots (34,41). This was evident in the radiological outcomes of this study. There were significant postoperative improvements in both the anterior and posterior disc heights, which remained radiologically evident at 12 months follow-up. Similarly, the significant improvement in spondylolisthesis was also maintained at 12 months follow-up. This supports the evidence that DLIF, by both utilizing large implants and preserving the stabilizing spinal ligaments, which allows for ligamentotaxis, corrects spinal alignment (34,41,42).
Despite the literature supporting the effectiveness of DLIF in improving segmental lordosis at the operative level, there is a lack of studies that show an improvement in lumbar lordosis (18,21,43,44). Consistent with the literature, our results failed to show any significant postoperative differences in the lumbar lordosis. This finding may be consequent to several factors. First, it is plausible that because PEEK cages with a lordotic angle of 0° were utilized, they may be less likely to improve lumbar lordosis. However, Lee et al. investigated the effect of the lordotic angle of PEEK cages on lumbar lordosis in patients undergoing one level PLIF and found no significant improvement in lumbar lordosis with increasing lordotic angle (45). Second, given that a significant proportion of those included in the study were older patients and likely to have poor bone quality, significant screw compression during instrumentation with pedicle screw fixation, which forms lordosis, was not applied in order to attempt to minimize subsidence. Third, the majority of DLIFs performed in this study were in the mid-lumbar region, which contributes less significantly to lumbar lordosis compared to L4/5 and L5/S1. Finally, given that the surgical approach does not involve sectioning the anterior longitudinal ligament or removal of the facet joint, this may limit the utility of DLIF in increasing lumbar lordosis.
Significant improvement in local disc angle was observed postoperatively at 6 weeks and 6 months but was no longer evident at 12 months follow up. This may be due to the several factors. Based on the anterior and posterior disc heights, although still statistically significant at 1 year, there is a degree of subsidence that is evident from 6 months postoperatively, which may explain the change in local disc angle. Furthermore, PEEK cages with a lordotic angle of 0° were used, which together with subsidence, may account for the loss of disc angle. However, given that few studies report postoperative local disc angles, it is difficult to compare our results with the current literature. A recent study by Kim et al. also reported significant changes in disc angle, however, postoperative measurements were only conducted at 6 days postoperatively (46). As such, the results at 1 year postoperatively cannot be compared. Nonetheless, our findings are supported by previous literature, which report that DLIF, although effective for correction of Cobb angle and restoration of segmental lordosis and disc height, is not effective for the restoration of lumbar lordosis and sagittal alignment (18,43,47).
Given the significant improvement in postoperative disc heights, indirect decompression was evident in our study. The significant increase in foraminal height and width at 6 weeks and 6 months postoperatively was also indicative of effective indirect decompression. These results are comparable to studies with reduced time to follow-up. For example, Oliveira et al. reported a significant increase in the foraminal height postoperatively (13.5% increase in foraminal height, P=0.0027), however, this study only obtained measurements at up to 2 weeks postoperatively (34). However, our results failed to demonstrate significant long-term improvement in foraminal height and width. This is in contrast to several studies that have demonstrated effective foraminal decompression at long-term follow-up (≥6 months) (20,21,48).
In light of the findings of previous studies, the inability of this study to demonstrate long-term indirect foraminal decompression may be attributable to a lack of statistical power. Not all of the 50 included patients had postoperative imaging at 12 months, as it is not standard postoperative care of the surgeon (GD) to re-image asymptomatic patients if satisfactory radiographic results are evident at 6 months follow-up. It should be noted that this is also a plausible explanation for the fact that the local disc angle was not significantly maintained at 12 months follow-up. Furthermore, the subsidence that was evident from 6 months postoperatively may account for the loss of foraminal decompression. Lee et al. examined the radiological outcomes between TLIF and DLIF and noted that DLIF had a greater corrective force with respect to both disc and foraminal heights (20). This was partially attributed to the difference between the cages utilized, including a taller cage height used in the DLIF group. Interestingly, the mean cage height utilized in this DLIF group was 12.94±1.30 mm compared to our mean PEEK cage height, which was 11.39±1.58 mm. As such, it is plausible that subsidence, combined with the short cage height used, accounts for the loss of foraminal decompression. Nevertheless, despite the loss of foraminal height and width at 12 months postoperatively, the long-term clinical outcome scores (mean postoperative follow-up time of 2.1±1.2 years) were indicative of long-term effective indirect decompression.
In our study, fusion was primarily assessed using dynamic plain radiographs. In the Australian healthcare environment standard clinical practise is to only use CT scanning to assess fusion if there is a clinical suspicion of mal-union. At 6 and 12 months postoperatively, fusion was achieved in 62.2% and 89.2% of patients, respectively. These rates of fusion were comparable to those reported in the literature. For example, Lee et al. reported a fusion rate of 60.9% and 87.8% at 6 and 12 months postoperatively, respectively (20). Similarly, Malham et al. determined the rate of fusion at 6 and 12 months postoperatively to be 46% and 85%, respectively (49). Rodgers et al. reported a rate of fusion of 96.6% at 12 months postoperatively. A probable explanation for this higher rate of fusion can be accounted for by the fact that 12-month postoperative CT scans were carried out for all patients included in the study, thus allowing for a more accurate assessment of fusion (50). Compared to more traditional approaches, our study demonstrates similar fusion rates. A recent systematic review and meta-analysis by Ajiboye et al. found the fusion rate for ALIF to be between 80.0–95.8% and between 77.0–95.2% for TLIF (51). The slightly higher rates of fusion reported are likely accounted for by the fact that the mean follow-up time for the studies included in the systematic review were greater than 12 months.
As discussed, the DLIF approach mitigates many of the vascular complications and bony resections associated with other minimally invasive techniques (10-16,52). However, visualization through smaller incisions is difficult and the use of dilators to access the disc space puts the psoas muscle and nerves of the lumbar plexus at risk of injury. Consequently, there are concerns regarding postoperative complications, in particular, neural and psoas muscle injuries (19,22,24-27). Minor complications including: anterior thigh pain, numbness and/or dysesthesias, as well as hip flexor weakness, have been reported in 19–67% of patients whom have undergone DLIF (24,26,27). This variability in reported complication rate is likely consequent to outcome data extrapolated from different surgeons, at different institutions, using different surgical techniques.
To address the relative paucity of literature pertaining to postoperative complications, Grimm et al. recently reviewed 108 patients who underwent lateral interbody fusion procedures at a single institution. They noted 25 complications (23%), of which, 4 patients (3.7%) experienced major complications including persistent stenosis, vertebral body fracture, contralateral nerve root injury and dense quadriceps paresis (25). Twenty-one patients (19.4%) had minor complications primarily consequent to approach-related thigh-pain and/or paresthesia and hip flexor weakness (25). Two of the 21 patients experienced DVT after undergoing multilevel procedures (25). These complication rates are consistent with those reported by Knight et al. (22.4%), of which, 13.8% were related to surgical approach (19). The largest study to date by Rodgers et al. reported a significantly lower complication rate with only 4 of 600 patients (0.7%) experiencing transient postoperative neural deficits (22). However, this markedly lower complication rate may be attributed to the fact that, in contrast with other studies, Rodgers et al. did not classify ipsilateral thigh numbness or hip flexor weakness to be surgical complications. Rather, these phenomena were considered practically inevitable postoperative outcomes (22).
The overall DLIF complication rate was lower in our study population relative to those described by both Grimm et al. and Knight et al. (12% versus 23% and 22.4%, respectively) (19,25). Approach-related injuries (6%) were significantly lower than those reported in the literature and of the 12% overall complication rate, all were transient and no major complications occurred. The sensory neural and psoas muscle symptoms, had entirely resolved between 8 to 16 weeks postoperatively, which was earlier than recovery times reported by Grimm et al. where symptoms had resolved between 3–6 months postoperatively (25). Nonetheless, symptom resolution is consistent with the literature, which reports that 50–84% of these complications resolved within 6 months (24,26,27). It is important to note that DLIF is known to have a steep learning curve, with a reduction in complication rate occurring over time, as the surgeon becomes more adept in the surgical technique (19,21,25,41). This phenomenon was not observed in this study. The complication rate was stable throughout the 4-year review period and complications occurred equally in single and multi-level operations.
In all DLIFs performed, neural complications were minimized by placing EMG electrodes in the L2–L5 myotomes and continuous intraoperative monitoring was utilized to minimize injury to the lumbar plexus and ensure neural elements were not at risk across the operative field. Furthermore, to minimize damage to the lumbar plexus, which migrates ventrally from L1 to L5 within the psoas muscle, the surgical approach to the disc space involved placement of the dilators and retractor as anteriorly as possible, particularly when operating on the L4/5 level (53). Vascular injuries were avoided by careful preoperative evaluation of the arterial and venous anatomy to ensure a safe working corridor, and at L4/5 this would often necessitate a left sided approach to avoid the right common iliac vein. It was also considered important that the surgeon stand anterior to the patient during the procedure, so that instruments were directed away from the anterior vascular structures, particularly during the discectomy.
Limitations
A number of limitations can be identified in this study. First, the study included a small sample size, thereby reducing the external validity of the results yielded. Second, this study is a single surgeon series and may reflect the technical skill of the surgeon and may not be applicable in general. Third, there were a variety of sources of heterogeneity that could not be accounted for. This included variation in indication for surgery, which included degenerative and deformity, although given the small sample size we were not able to perform subgroup analysis. There was also heterogeneity due to mixed cases of single-level versus multi-level fusions, and we did not assess the impact of baseline characteristics in terms of influencing on reported outcomes. Future studies can mitigate this by performing multivariate-adjusted analysis when comparing DLIF to other interbody fusion techniques. Finally, clinician concern pertaining to unnecessary radiation exposure resulted in a number of patients not undergoing medical imaging at 12 months follow-up. However, we believe that the results obtained are indicative of a realistic clinical scenario wherein multiple imaging modalities are employed and follow-up times are inconsistent.
Conclusions
This study demonstrates encouraging medium-term clinical and radiological results for DLIF. DLIF was found to significantly improve VAS back pain scores and reduce ODI and RMDQ scores. Similarly, DLIF was determined to have comparable rates of fusion to more traditional approaches and significantly improve a number of radiological outcomes, including spondylolisthesis, disc height, local disc angle and foraminal height and width. However, the ability of DLIF to improve local disc angle and decompress the foramen was not demonstrated at 12 months. Future studies should include prospective, multi-centre, adequately powered studies with long-term follow-up to allow for an improved analysis of these radiological outcomes. Finally, DLIF proved to be a safe procedure with a low complication rate.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Institutional human ethics approval was obtained prior to commencement of the review (LNR/14/WMEAD/425).
References
- Berjano P, Balsano M, Buric J, et al. Direct lateral access lumbar and thoracolumbar fusion: preliminary results. Eur Spine J 2012;21 Suppl 1:S37-42. [Crossref] [PubMed]
- Ohtori S, Koshi T, Suzuki M, et al. Uni- and bilateral instrumented posterolateral fusion of the lumbar spine with local bone grafting: a prospective study with a 2-year follow-up. Spine (Phila Pa 1976) 2011;36:E1744-8. [Crossref] [PubMed]
- Zhou ZJ, Zhao FD, Fang XQ, et al. Meta-analysis of instrumented posterior interbody fusion versus instrumented posterolateral fusion in the lumbar spine. J Neurosurg Spine 2011;15:295-310. [Crossref] [PubMed]
- Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine 2003;28:S26-S35. [Crossref] [PubMed]
- Phan K, Hogan JA, Mobbs RJ. Cost-utility of minimally invasive versus open transforaminal lumbar interbody fusion: systematic review and economic evaluation. Eur Spine J 2015;24:2503-13. [Crossref] [PubMed]
- Phan K, Rao PJ, Kam AC, et al. Minimally invasive versus open transforaminal lumbar interbody fusion for treatment of degenerative lumbar disease: systematic review and meta-analysis. Eur Spine J 2015;24:1017-30. [Crossref] [PubMed]
- Schwender JD, Holly LT, Rouben DP, et al. Minimally Invasive Transforaminal Lumbar Interbody Fusion (TLIF): Technical Feasibility and Initial Results. J Spinal Disord Tech 2005;18 Suppl:S1-6. [Crossref] [PubMed]
- Starkweather AR, Witek-Janusek L, Nockels RP, et al. The Multiple Benefits of Minimally Invasive Spinal Surgery: Results Comparing Transforaminal Lumbar Interbody Fusion and Posterior Lumbar Fusion. J Neurosci Nurs 2008;40:32-9. [Crossref] [PubMed]
- Phan K, Rao PJ, Scherman DB, et al. Lateral lumbar interbody fusion for sagittal balance correction and spinal deformity. J Clin Neurosci 2015;22:1714-21. [Crossref] [PubMed]
- Baker JK, Reardon PR, Reardon MJ, et al. Vascular injury in anterior lumbar surgery. Spine 1993;18:2227-30. [Crossref] [PubMed]
- Rajaraman V, Vingan R, Roth P, et al. Visceral and vascular complications resulting from anterior lumbar interbody fusion. J Neurosurg 1999;91:60-4. [PubMed]
- Rihn JA, Patel R, Makda J, et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J 2009;9:623-9. [Crossref] [PubMed]
- Smith WD, Christian G, Serrano S, et al. A comparison of perioperative charges and outcome between open and mini-open approaches for anterior lumbar discectomy and fusion. Journal of Clinical Neuroscience 2012;19:673-80. [Crossref] [PubMed]
- Tiusanen H, Seitsalo S, Österman K, et al. Retrograde ejaculation after anterior interbody lumbar fusion. Eur Spine J 1995;4:339-42. [Crossref] [PubMed]
- Villavicencio AT, Burneikiene S, Bulsara KR, et al. Perioperative complications in transforaminal lumbar interbody fusion versus anterior–posterior reconstruction for lumbar disc degeneration and instability. J Spinal Disord Tech 2006;19:92-7. [Crossref] [PubMed]
- Wood KB, DeVine J, Fischer D, et al. Vascular injury in elective anterior lumbosacral surgery. Spine 2010;35:S66-S75. [Crossref] [PubMed]
- Kim JS, Lee HS, Shin DA, et al. Correction of Coronal Imbalance in Degenerative Lumbar Spine Disease Following Direct Lateral Interbody Fusion (DLIF). Korean J Spine 2012;9:176-80. [Crossref] [PubMed]
- Acosta FL Jr, Liu J, Slimack N, et al. Changes in coronal and sagittal plane alignment following minimally invasive direct lateral interbody fusion for the treatment of degenerative lumbar disease in adults: a radiographic study: Clinical article. J Neurosurg Spine 2011;15:92-6. [Crossref] [PubMed]
- Knight RQ, Schwaegler P, Hanscom D, et al. Direct lateral lumbar interbody fusion for degenerative conditions: early complication profile. J Spinal Disord Tech 2009;22:34-7. [Crossref] [PubMed]
- Lee YS, Kim YB, Park SW, et al. Comparison of transforaminal lumbar interbody fusion with direct lumbar interbody fusion: clinical and radiological results. J Korean Neurosurg Soc 2014;56:469-74. [Crossref] [PubMed]
- Lee YS, Park SW, Kim YB. Direct lateral lumbar interbody fusion: clinical and radiological outcomes. J Korean Neurosurg Soc 2014;55:248-54. [Crossref] [PubMed]
- Rodgers WB, Gerber EJ, Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: an analysis of 600 cases. Spine 2011;36:26-32. [Crossref] [PubMed]
- Youssef JA, McAfee PC, Patty CA, et al. Minimally invasive surgery: lateral approach interbody fusion: results and review. Spine 2010;35:S302-S311. [Crossref] [PubMed]
- Cummock MD, Vanni S, Levi AD, et al. An analysis of postoperative thigh symptoms after minimally invasive transpsoas lumbar interbody fusion: clinical article. J Neurosurg Spine 2011;15:11-8. [Crossref] [PubMed]
- Grimm BD, Leas DP, Poletti SC, et al. Postoperative complications within the first year after extreme lateral interbody fusion: Experience of the first 108 patients. Clin Spine Surg 2016;29:E151-6. [Crossref] [PubMed]
- Le TV, Burkett CJ, Deukmedjian AR, et al. Postoperative lumbar plexus injury after lumbar retroperitoneal transpsoas minimally invasive lateral interbody fusion. Spine 2013;38:E13-E20. [Crossref] [PubMed]
- Moller DJ, Slimack NP, Acosta FL Jr, et al. Minimally invasive lateral lumbar interbody fusion and transpsoas approach-related morbidity. Neurosurg Focus 2011;31:E4. [Crossref] [PubMed]
- Bina RW, Zoccali C, Skoch J, et al. Surgical anatomy of the minimally invasive lateral lumbar approach. J Clin Neurosci 2015;22:456-9. [Crossref] [PubMed]
- Guérin P, Obeid I, Bourghli A, et al. The lumbosacral plexus: anatomic considerations for minimally invasive retroperitoneal transpsoas approach. Surg Radiol Anat 2012;34:151-7. [Crossref] [PubMed]
- Avila MJ, Walter CM, Baaj AA. Outcomes and Complications of Minimally Invasive Surgery of the Lumbar Spine in the Elderly. Cureus 2016;8:e519. [PubMed]
- Fehlings MG, Tetreault L, Nater A, et al. The aging of the global population: the changing epidemiology of disease and spinal disorders. Neurosurgery 2015;77:S1-S5. [Crossref] [PubMed]
- Pannell WC, Savin DD, Scott TP, et al. Trends in the surgical treatment of lumbar spine disease in the United States. Spine J 2015;15:1719-27. [Crossref] [PubMed]
- Podichetty VK. The aging spine: the role of inflammatory mediators in intervertebral disc degeneration. Cell Mol Biol (Noisy-le-grand) 2007;53:4-18. [PubMed]
- Oliveira L, Marchi L, Coutinho E, et al. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine 2010;35:S331-S337. [Crossref] [PubMed]
- DiPaola CP, Molinari RW. Posterior lumbar interbody fusion. Journal of the American Academy of Orthopaedic Surgeons 2008;16:130-9. [Crossref] [PubMed]
- Phan K, Ramachandran V, Tran T, et al. Impact of Elderly Age on Complications and Clinical Outcomes following Anterior Lumbar Interbody Fusion Surgery. World Neurosurg 2017;105:503-9. [Crossref] [PubMed]
- Phan K, Rogers P, Rao PJ, et al. Influence of obesity on complications, clinical outcome and subsidence following anterior lumbar interbody fusion (ALIF): prospective observational study. World Neurosurg 2017;107:334-41. [Crossref] [PubMed]
- Phan K, Thayaparan GK, Mobbs RJ. Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion--systematic review and meta-analysis. Br Br J Neurosurg 2015;29:705-11. [Crossref] [PubMed]
- Rao PJ, Phan K, Giang G, et al. Subsidence following anterior lumbar interbody fusion (ALIF): a prospective study. J Spine Surg 2017;3:168-75. [Crossref] [PubMed]
- Teng I, Han J, Phan K, et al. A meta-analysis comparing ALIF, PLIF, TLIF and LLIF. J Clin Neurosci 2017;44:11-7. [Crossref] [PubMed]
- Ozgur BM, Aryan HE, Pimenta L, et al. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 2006;6:435-43. [Crossref] [PubMed]
- Dooris AP, Goel VK, Grosland NM, et al. Load-sharing between anterior and posterior elements in a lumbar motion segment implanted with an artificial disc. Spine 2001;26:E122-E129. [Crossref] [PubMed]
- Johnson RD, Valore A, Villaminar A, et al. Pelvic parameters of sagittal balance in extreme lateral interbody fusion for degenerative lumbar disc disease. J Clin Neurosci 2013;20:576-81. [Crossref] [PubMed]
- Malham GM, Ellis NJ, Parker RM, et al. Maintenance of Segmental Lordosis and Disk Height in Stand-alone and Instrumented Extreme Lateral Interbody Fusion (XLIF). Clin Spine Surg 2017;30:E90-E98. [Crossref] [PubMed]
- Lee JH, Lee DO, Lee JH, et al. Effects of lordotic angle of a cage on sagittal alignment and clinical outcome in one level posterior lumbar interbody fusion with pedicle screw fixation. Biomed Res Int 2015;2015:523728. [PubMed]
- Kim SJ, Lee YS, Kim YB, et al. Clinical and radiological outcomes of a new cage for direct lateral lumbar interbody fusion. Korean J Spine 2014;11:145-51. [Crossref] [PubMed]
- Tormenti MJ, Maserati MB, Bonfield CM, et al. Complications and radiographic correction in adult scoliosis following combined transpsoas extreme lateral interbody fusion and posterior pedicle screw instrumentation. Neurosurgical Focus 2010;28:E7. [Crossref] [PubMed]
- Kepler CK, Sharma AK, Huang RC, et al. Indirect foraminal decompression after lateral transpsoas interbody fusion. J Neurosurg Spine 2012;16:329-33. [Crossref] [PubMed]
- Malham GM, Ellis NJ, Parker RM, et al. Clinical outcome and fusion rates after the first 30 extreme lateral interbody fusions. ScientificWorldJournal 2012;2012:246989. [Crossref] [PubMed]
- Rodgers WB, Gerber EJ, Patterson JR. Fusion after minimally disruptive anterior lumbar interbody fusion: Analysis of extreme lateral interbody fusion by computed tomography. SAS J 2010;4:63-6. [Crossref] [PubMed]
- Ajiboye RM, Alas H, Mosich GM, et al. Radiographic and Clinical Outcomes of Anterior and Transforaminal Lumbar Interbody Fusions: A Systematic Review and Meta-analysis of Comparative Studies. Clin Spine Surg 2018;31:E230-E238. [Crossref] [PubMed]
- Phan K, Xu J, Scherman DB, et al. Anterior Lumbar Interbody Fusion With and Without an "Access Surgeon": A Systematic Review and Meta-analysis. Spine (Phila Pa 1976) 2017;42:E592-E601. [Crossref] [PubMed]
- Davis TT, Bae HW, Mok MJM, et al. Lumbar plexus anatomy within the psoas muscle: implications for the transpsoas lateral approach to the L4-L5 disc. J Bone Joint Surg Am 2011;93:1482-7. [Crossref] [PubMed]


