Is the routine use of magnetic resonance imaging indicated in patients with scoliosis?
Introduction
The association between spinal cord abnormalities and spinal deformity is well established (1). The continued improvement and availability of more specialist and detailed imaging has meant that the actual prevalence of underlying spinal cord abnormalities is only now being appreciated (2). However, many of these abnormalities are subclinical and the patient may have no associated signs or symptoms. As a result, the need for routine magnetic resonance imaging (MRI) of the whole spine is debated (3). Many institutions rely on certain criteria, either from the history, clinical examination or plain radiographic findings, in determining whether further imaging is indicated. Other institutions, such as ours, perform whole spine MRI in all patients referred to the spinal deformity service as part of routine care to rule out any neural axis abnormality (NAA) that may alter management or change the consent process.
The aim of this study was to perform a retrospective review of the MRI findings, both intra- and extraspinal, of all patients presenting to the spinal deformity service at our institution. This is to determine if the indications for MRI as stated in the literature are indeed true indicators of underlying abnormalities and to also identify the incidence of incidental extra-spinal findings.
Methods
All patients referred to our centre with scoliosis undergo routine neurological examination and plain film radiography (whole spine PA and lateral views in the standing position). MRI is performed as routine care in all patients, provided there is no contraindication and regardless of whether surgical intervention is undertaken, to ensure that there is no underlying NAA that may alter the treatment pathway away from what is otherwise expected. All MRIs are reported by consultant radiologists with a special interest in musculoskeletal radiology and spinal deformity. Any MRI abnormalities are identified and referred on to the appropriate specialty as required. Corrective procedures for scoliosis are then undertaken once all such anomalies had been investigated and managed.
The MRI reports of all patients under the age of 18 who underwent a whole spine MRI between January 2009 and February 2014 were reviewed by 3 assessors. The scoliosis was also assessed from a contemporaneous radiograph. The findings were then analysed by age, subdividing the group in to early onset and adolescent scoliosis groups as defined by Skaggs et al. (4). The details recorded included patient demographics, curve pattern, intra- and extraspinal abnormalities. The inclusion criteria were all patients who underwent whole spine MRI between the ages of 1 and 18 years for investigation of their scoliosis at our institution. Statistical analysis using the Fisher exact test was conducted with P<0.05.
Results
There were 1,008 patients who underwent whole spine MRI at our institution during the study period. Of these, 851 underwent MRI scans for investigation of their scoliosis and were included in our study. The average age was 14.08 years (SD =2.34). There were 211 male patients and 640 female patients. A breakdown of the abnormalities found can be seen in Tables 1,2.
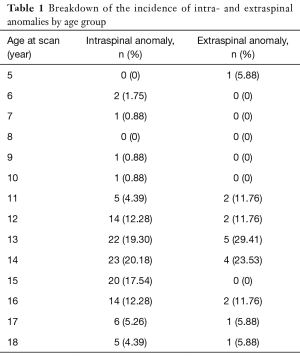
Full table
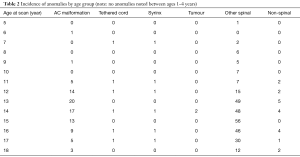
Full table
Neural axis abnormality
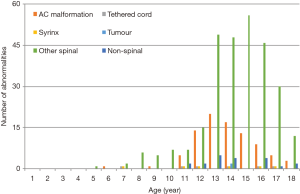
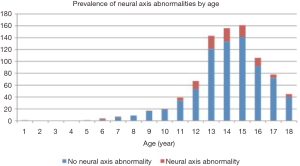
The incidence of NAAs in our population was 13.4% with 143 intraspinal abnormalities in 114 patients. The most common abnormality seen were AC malformations (n=88, 10.3%), and the presence of a syrinx (n=47, 5.5%). The prevalence of all abnormalities in our series can be seen in Table 3. The majority of these were in the adolescent age group as can be seen in Figures 1,2.
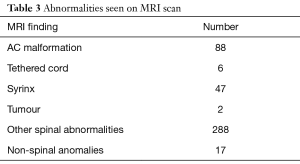
Full table
Neural axis abnormalities indicators
Age
Diagnosis before the age of 10 was found in only a small proportion of the population (n=39, 4.58%). Four of these children were found to have a NAA. When compared to the remainder of the population, this was found not to be statistically significant (P=0.81). The incidence of NAAs was found to be proportionally higher in the adolescent population in our series (Table 2). This is likely to be due to the fact that they represented a larger proportion of the population. Again, this was not found to be statistically significant (P=0.25).
Left thoracic curve
There were 49 patients in our population who had a left thoracic curve (5.76%). Five of these patients were in the early onset population, but none of them had an underlying NAA. Of the remaining patients, 8 were found to have NAAs. There were six patients who had a syrinx, one patient who had an AC malformation and one patient who had a tethered cord and cerebellar tonsillar descent. Having a left-sided thoracic curve was not statistically significant for the presence of an NAA (P=0.23) compared to other curve patterns.
Double thoracic curve
There were 13 double thoracic curves in our population with 1 NAA in this population (a small syrinx). Two of these were in the early onset group and 11 in the adolescent age group. The patient with the NAA abnormality was in the adolescent population. The presence of a double thoracic curve was not found to be significant in either population (P=1.00).
Gender
There were 14.2% (n=30) and 13.1% (n=84) of the overall male and female patients, respectively, found to have a NAA. Being male was not found to be statistically significant for the presence of a NAA (P=0.91)
Other spinal abnormalities
Of the cohort, 33.7% of patients, overall, demonstrated other spinal abnormalities. A total of 538 abnormalities (Table 4) were seen in 288 patients. The most commonly seen pathology was a disc anomaly in 145 patients (50.3%). There were 68 patients who also demonstrated a sagittal plane change although the nature of this was not always characterized. As one may expect given the focus of this study, a proportion of patients also demonstrated congenital anomalies (n=112, 38.9%).
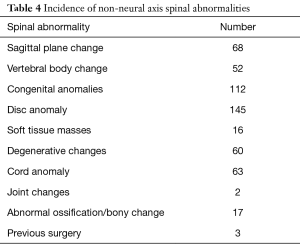
Full table
Extraspinal abnormalities
There were 17 patients found to have extraspinal abnormalities. Fourteen of these patients were female and the average age of this cohort was 13.8 years. The most common region for extraspinal abnormalities was the abdominal and pelvic region within the renal system and/or ovarian system as can be seen in Table 5. Three of these patients were also found to have NAAs.
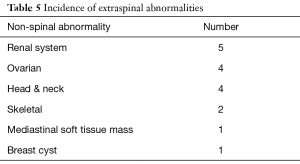
Full table
Discussion
The first presentation of patients with a NAA may be to a spinal deformity surgeon. The association between scoliosis and syringomyelia has been established since the mid-1940s (1). Subsequent work by Evans et al. (5) has shown that the incidence of syringomyelia or an Arnold Chiari malformation with scoliosis is 22.6%, whereas in another series it was just under 50% (6).
The concern in performing corrective surgery in patients with a NAA is whether there is an increased risk of neurological complications. Caution may be required in these patients as correction may result in the development of a neurological deficit (7). This may be because of changes in the anatomy of the canal causing damage to the spinal cord, threat to the vascularity of the spinal cord (8), changes in the cerebrospinal fluid pressure (9) or increased traction on already altered neural tissue (10). The evidence, however, is inconclusive. Whilst some support this theory, there is recently published evidence that the presence of NAA is not associated with an increased complication rate (11).
Diagnosis of such NAAs relies on MRI cross sectional imaging of the neural axis but the evidence for the routine usage of pre-operative MRI is not clear. In some studies, the prevalence of abnormalities has been reported as low as 2–4% (2,12). These studies were comparatively small in size than others published. Our study, as with Inoue’s study (13), suggests that the true incidence is higher than that.
There is some consensus regarding the indications for a pre-operative MRI. It is universally acknowledged that abnormal neurological examination is an indication for MRI. However, it has also been shown that a high proportion of patients with a normal neurological examination have an underlying NAA (14-16). The other indications for a pre-operative MRI are: early onset (5,14), atypical curvature (17,18), rapid progression (19), double thoracic curve (2) and male gender (20). We performed statistical analysis on these factors wherever possible in this study.
A right-sided thoracic curve in isolation or with a left lumbar curve have been described as ‘typical’ (12). A left-sided thoracic curve is, therefore, regarded as ‘atypical’ and an indication to perform an MRI scan. Of the 49 patients in our study that had this ‘atypical’ curve, 9 NAAs were identified in 8 patients. However, as with other studies, there was no statistically significant association between curve pattern and the presence of a NAA. This was also the case for the presence of a double thoracic curve.
Early onset scoliosis (EOS) is defined as diagnosis of scoliosis before the age of 10 by the Scoliosis Research Society (4). EOS was again not found to be statistically significant for the presence of a NAA. No other age group was found to be statistically significant either. This was also the case with respect to the sex of the patient. Being male is routinely used as an indication for performing MRI, but this was not found to be statistically significant in our population. Interestingly, in a subgroup of 3 male children, under the age of 10 with an ‘atypical’ curve, none were found to have an underlying NAA.
The cost of performing routine pre-operative MRI scans is one of the reasons it is not done in all centres. The only routine imagining modality used are plain radiographs. Thoracic apical segment lordosis is a feature on the lateral view that has been shown to be associated with and linked to adolescent idiopathic scoliosis (AIS) (21-23). Hence, its absence is regarded as pathognomonic of a NAA (24). However, the adequacy of these lateral radiographs is often lacking and as a result can be difficult to pick up (24). Reliance on this as a means of determining the presence of a NAA is ill-advised.
A further reason for the reluctance in performing routine MRIs, is that advances in MRI may have resulted in an over diagnosis of NAAs that are subclinical. Often no neurosurgical intervention is required or recommended. The relevance of these lesions and the risk associated with performing corrective surgery has never been truly established (5). There are reports of stabilization of the curve, prevention of further progression, and even resolution, once an underlying abnormality has been addressed (25,26). But, this improvement is generally only seen in those below the age of 10 years or with a flexible curve (18,26). Brockmeyer (27) in his study found that whilst a significant number in this age group do show an improvement following syrinx decompression, those over the age of 12 had a decreased chance. This may be due to permanent structural changes to the spine occurring as the child reaches skeletal maturity (28), believed to have been caused by the muscle imbalance produced by the compromise of the cells responsible for muscle trunk balance (7). Nevertheless, there is good evidence that there would be an improvement in any underlying neurological deficit that without an MRI scan may have been attributed to the spinal deformity (29). The authors of this study were, however, keen to point out that improvement did not equate to complete resolution nor normalization.
The whole spine protocol also raises the possibility of identifying an incidental lesion. An incidental lesion is one that is found whilst examining patient for an unrelated reason (30). These incidental findings (IFs) may be more significant than the reason for the original examination (31). The majority of these were in the abdominopelvic region as one would expect given the large number of organs present within this area. Since the introduction of PACS, there has been an increase in the number of IFs (30). The significance of these findings is difficult to determine and clinical correlation is required. Inevitably, some findings may be more significant than others and, in some circumstances, may account for the patient’s symptoms. Malignancies may be identified and those that are identified ‘incidentally’ have a superior prognosis and survival rate (32,33).
Whilst not a reason to perform a routine MRI, identification of such extraspinal findings is of paramount importance not only for the reasons above, but also in view of the fact that the they are a major source of litigation. A retrospective review of 18,860 lawsuits found that 47% of radiology lawsuits were related to missed diagnoses (34). in America and 60% of claims against radiologists in an Italian study (35,36). In order to aid radiologists in the detection and reporting of such IFs, the European Society of Radiology has issued specific guidance (37).
IFs in lumbar spine MRIs have been reported as high as 68.6% when using a systematic method of reporting (38). Most papers have, however, shown a much lower rate when performing a retrospective review of the original radiology report of between 7–8% (30,38,39). The incidence in our study represented 2.34% of the overall population. This may in part be explained that our paper looks exclusively at a paediatric and adolescent population. Some papers also report anatomical variations as IFs which may artificially inflate their incidence. Another plausible explanation for the wide variation in the reported IFs relates to the fact that the focus of the reporting radiologist will be on the spinal disease or complaint and it may have been reported by a radiologist with an interest in the musculoskeletal system. As a result, they may not recall much of their general training that may be necessary for determining the presence of extraspinal pathology (38). Nevertheless, caution must also be exercised in the manner in which these lesions are reported. The vast majority of IFs are benign and may result in unnecessary medical costs and anxiety. Therefore, the communication, discussion and reporting of any IFs should be conducted in a sensitive manner.
The weaknesses of this study are that it has not proved possible to analyse the data further for other associations of NAA and curve behaviour (progressive versus not progressive), curve size (small versus large) or those that did or did not go on to surgical intervention due to the small numbers of NAAs identified. A larger sample would be required for this. It has not been possible to identify whether any of these anomalies required intervention separate to the management of the scoliosis.
Conclusions
The question when performing any investigation is how it could alter management. The evidence with respect to routine pre-operative MRI, scoliosis and underlying NAA is mixed. There appears to be no real consensus whether it would alter the outcome in these patients, leave them with an increased risk of complication or influence the progression of their scoliosis. What is clear from our study is that the incidence may well be greater than has been previously quoted. Furthermore, many of the indications used for performing an MRI of the spine cannot be regarded as genuine predictors of an underlying NAA and, therefore, they cannot be relied upon. The data presented here acts as normative values for the incidence of both neural axis anomalies and extra spinal anomalies and in a large population of children with scoliosis at different ages.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was from data collected as part of an institutional audit of previously collected, anonymized data from standard care. In this circumstance there is no requirement in the UK for ethical approval or consent. The audit did have Institutional Review Board approval as per the policies and procedures of the institution.
References
- Woods WW, Pimenta AM. Intramedullary lesions of the spinal cord. Arch Neurol Psychiatr 1944;52:383. [Crossref]
- Maiocco B, Deeney VF, Coulon R, et al. Adolescent idiopathic scoliosis and the presence of spinal cord abnormalities. Preoperative magnetic resonance imaging analysis. Spine 1997;22:2537-41. [Crossref] [PubMed]
- Kim H, Kim HS, Moon ES, et al. Scoliosis Imaging: What Radiologists Should Know. Radiographics 2010;30:1823-42. [Crossref] [PubMed]
- Skaggs D, Guillaume T, El-Hawary R, et al. Early onset scoliosis consensus statement, SRS growing spine committee 2015. Spine Deformity 2015;3:107. [Crossref]
- Evans SC, Edgar MA, Hall-Craggs MA, et al. MRI of 'idiopathic' juvenile scoliosis. A prospective study. J Bone Joint Surg Br 1996;78:314-7. [Crossref] [PubMed]
- Milhorat TH, Chou MW, Trinidad EM, et al. Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery 1999;44:1005-17. [Crossref] [PubMed]
- Huebert HT, MacKinnon WB. Syringomyelia and scoliosis. J Bone Joint Surg Br 1969;51:338-43. [Crossref] [PubMed]
- Phillips WA, Hensinger RN, Kling TF Jr. Management of scoliosis due to syringomyelia in childhood and adolescence. J Pediatr Orthop 1990;10:351-4. [Crossref] [PubMed]
- Tomlinson RJ Jr, Wolfe MW, Nadall JM, et al. Syringomyelia and developmental scoliosis. J Pediatr Orthop 1994;14:580-5. [Crossref] [PubMed]
- Bradley LJ, Ratahi ED, Crawford HA, et al. The outcomes of scoliosis surgery in patients with syringomyelia. Spine (Phila Pa 1976) 2007;32:2327-33. [Crossref] [PubMed]
- Noordeen MH, Taylor BA, Edgar MA. Syringomyelia: a potential risk factor in scoliosis surgery. Spine 1994;19:1406-9. [Crossref] [PubMed]
- Do T, Fras C, Burke S, Widmann RF, et al. Clinical value of routine preoperative magnetic resonance imaging in adolecscent idiopathic scoliosis. A prospective study of three hundred and twenty-seven patients. J Bone Joint Surg Am 2001;83:577-9. [Crossref] [PubMed]
- Inoue M, Minami S, Nakata Y, et al. Preoperative MRI analysis of patients with idiopathic scoliosis. Spine 2005;30:108-14. [Crossref] [PubMed]
- Lewonowski K, King JD, Nelson MD. Routine use of magnetic resonance imaging in idiopathic scoliosis patients less than eleven years of age. Spine 1992;17:S109-16. [Crossref] [PubMed]
- Gupta P, Lenke LG, Bridwell KH. Incidence of neural axis abnormalities in infantile and juvenile patients with spinal deformity. Spine 1998;23:206-10. [Crossref] [PubMed]
- Rajasekaran S, Kamath V, Kiran R, et al. Intraspinal anomalies in scoliosis: an MRI analysis of 177 consecutive scoliosis patients. Indian J Orthop 2010;44:57-63. [Crossref] [PubMed]
- Winter PD, Lonstein JE, Heithoff KB, et al. Magnetic resonance imaging evaluation of the adolescent patient with idiopathic scoliosis before instrumentation and fusion: a prospective, double blinded study of 140 patients. Spine 1997;22:855-8. [Crossref] [PubMed]
- Schwend RM, Henrikus W, Hall JE, et al. Childhood scoliosis: Clinical indications for magnetic resonance imaging. J Bone Joint Surg Am 1995;77:46-53. [Crossref] [PubMed]
- Baker AS, Dove J. Progressive scoliosis can be the first presenting sign of syringomyelia: a report of a case. J Bone Joint Surg Br 1983;65:472-3. [Crossref] [PubMed]
- Tokunaga M, Minami S, Isobe K, et al. Study on the scoliosis complicated with syringomyelia. Spinal Deformity 1997;22:855-88.
- Deacon P, Flood BM, Dickson RA. Idiopathic scoliosis in three dimensions. A radiographic and morphometric analysis. J Bone Joint Surg Br 1984;66:509-12. [Crossref] [PubMed]
- Dickson RA, Lawton JO, Archer IA, et al. The pathogenesis of idiopathic scoliosis. Biplanar spinal asymmetry. J Bone Joint Surg Br 1984;66:8-15. [Crossref] [PubMed]
- Howell FR, Dickson RA. The deformity of idiopathic scoliosis made visible by computer graphics. J Bone Joint Surg Br 1989;71:399-403. [Crossref] [PubMed]
- Davids JR, Chamberlin E, Blackhurst DW. Indications for Magnetic Resonance Imaging in Presumed Adolescent Idiopathic Scoliosis. J Bone joint Surg Am 2004;86:2187-95. [Crossref] [PubMed]
- Nakahara D, Yonezawa I, Kobanawa K, et al. Magnetic resonance imaging evaluation of patients with idiopathic scoliosis: a prospective study of four hundred seventy-two outpatients. Spine (Phila Pa 1976) 2011;36:E482-5. [Crossref] [PubMed]
- Yeom JS, Lee CK, Park KW, et al. Scoliosis associated with syringomyelia: analysis of MRI and curve progression. Eur Spine J. 2007;16:1629-35. [Crossref] [PubMed]
- Brockmeyer D, Gollogly S, Smith JT. Scoliosis associated with Chiari 1 malformations: the effect of suboccipital decompression on scoliosis curve progression: a preliminary study. Spine (Phila Pa 1976) 2003;28:2505-9. [Crossref] [PubMed]
- Eule JM, Erickson MA, O’Brien MF, et al. Chiari I Malformation Associated with Syringomyelia and Scoliosis A Twenty-Year Review of Surgical and Nonsurgical Treatment in a Pediatric Population. Spine 2002;27:1451-5. [Crossref] [PubMed]
- Park JK, Gleason PL, Madsen JR, et al. Presentation and management of Chiari I malformation in children. Pediatr Neurosurg 1997;26:190-6. [Crossref] [PubMed]
- Park HJ, Jeon YH, Rho MH, et al. Incidental findings of the lumbar spine at MRI during herniated intervertebral disk disease evaluation. AJR Am J Roentgenol 2011;196:1151-5. [Crossref] [PubMed]
- Westbrook JI, Braighwaite J, Mclntosh JH. The outcomes for patients with incidental lesions: serendipitous or iatrogenic? AJR Am J Roentgenol 1998;171:1193-6. [Crossref] [PubMed]
- Yamashita S, Oikawa K, Aizawa M, et al. Long-term prognosis of incidental renal cell carcinoma--clinical analysis of renal cell carcinoma detected by our health checkup. Nihon Hinyokika Gakkai Zasshi 2007;98:614-8. [Crossref] [PubMed]
- Palsdottir HB, Hardarson S, Petursdottir V, et al. Incidental detection of renal cell carcinoma is an independent prognostic marker: results of a long-term, whole population study. J Urol 2012;187:48-53. [Crossref] [PubMed]
- Berlin L, Berlin JW. Malpractice and radiologists in Cook County, Il: trends in 20 years of litigation. AJR Am J Roentgenol 1995;165:781-8. [Crossref] [PubMed]
- Fileni A, Magnavita N. A 12-year follow-up study of malpractice claims against radiologists in Italy. Radiol Med 2006;111:1009-22. [Crossref] [PubMed]
- Magnavita N, Magnavita G, Fileni A, et al. Ethical problems in radiology: medical error and disclosure. Radiol Med 2009;114:1345-55. [Crossref] [PubMed]
- European Society of Radiology (ESR). Good practice for radiological reporting. Guidelines from the European Society of Radiology (ESR). Insights Imaging 2011;2:93-6. [Crossref] [PubMed]
- Quattrocchi CC, Giona A, Di Martino AC, et al. Extra-spinal incidental findings at lumbar spine MRI in the general population: a large cohort study. Insights Imaging 2013;4:301-8. [Crossref] [PubMed]
- Wagner SC, Morrison WB, Carrino JA, et al. Picture archiving and communication system: effect on reporting of incidental findings. Radiology 2002;225:500-5. [Crossref] [PubMed]