
Editorial on “Can navigation-assisted surgery help achieve negative margins in resection of pelvic and sacral tumor?”
I enjoy reading the paper titled “Can navigation-assisted surgery help achieve negative margins in resection of pelvic and sacral tumor?” (1). It is a retrospective review of 23 navigation-assisted surgeries, which took place within a period of 6 years. The authors describe the histological margin of the specimens, the oncological outcome and the related complications.
As limb-sparing surgery (2) is becoming the gold standard in the management of pelvic and sacral bone tumors, precision in the bone osteotomy is one of the main concerns in how to achieve a satisfactory oncological outcome. On one hand, we would like to have a negative surgical margin (2,3) in order to minimize the risk of local tumor recurrence. On the other hand, a wider resection margin may remove more bone than is required. This makes reconstruction in the remaining bone much more difficult or indeed impossible. Therefore, we need a well-thought-out resection plan pre-operatively (4). This plan must be executed with reliability intra-operatively as described in this paper.
Pre-operative planning of the surgical margin
Enneking et al. (5) introduced the concept of intra-lesional, marginal, wide and radical margin in bone and soft tissue sarcoma resection surgery. Unfortunately, resection going through the normal tissue in wide margin resection does not share the same meaning in different sarcoma surgeons’ interpretations. Some would accept just outside the inflammatory zone whilst others only accept more than a certain distance, such as three centimeters from the inflammation zone. There is no gold standard to follow.
Kawaguchi et al. (3) tried to clarify the safety margin in excision of bone and soft tissue sarcoma. They advised that we should have the exact dimension in sub-classifying the “wide” resection margin histologically. They used the term “inadequate wide” for surgical specimens with normal tissue cuff of 1 centimeter and “adequate wide” for surgical specimens with normal tissue cuff of 2 to 4 centimeters. If it was of 5 or more than 5 centimeters, he would rename it “curative”. Although their statistics showed that the local recurrence rate was lower for the wider margin procedures, the authors did not mention the number of margin involvements in the pre-operatively planned “wide” or “curative” resections.
White et al. (6) meticulously analyzed soft tissue sarcoma resection specimens. They found that 10 out of 15 resected sarcomas had their tumor satellites in the inflammatory zone. Six of them were within 1 centimeter away from the main tumor. Four of them were more than 1 centimeter. Actually, there was one satellite at 4 centimeters away from the main tumor. In addition, 3 out of the 24 specimens taken from the non peri-tumoral edema areas either histologically or by the magnetic resonance imaging (MRI) scan images, showed positive tumor cells.
Therefore, if we have the surgical margin planned very narrowly in pre-operative planning, there is a high chance that we have margin involvement or satellite lesions left behind even though the bone cut is made exactly as preplanned. A poorly designed pre-operative plan cannot be compensated by a precisely executed surgery. Unfortunately, there is no consensus on the minimum margin distance. The optimum dimension of the surgical margin (cm) in planning depends on the nature of tumor, aggressiveness, pre-operative chemotherapy or radiotherapy and also according to the surgeon’s belief (4).
Meaning of negative margin in histology and local recurrence
There are two ways to prepare the resection margins of surgical specimen for histology: the shaved (en face) margin and the perpendicular margin. For shaved margin, the resection surface is shaved for microscopic assessment and the report is either “margin involved” or “margin not involved”. It has the advantage of assessing over a large surface area to determine whether the tumor has actually reached the resection margin. However, when the tumor is close to the margin, the exact distance between the tumor and the margin cannot be accurately studied microscopically. For tumor anticipated to be close to the resection margin, the perpendicular margin is advised (7). The specimen is sectioned along a plane where the tumor is expected to be the closest to the resection margin, and the closest distance between the tumor and the resection margin can then be measured microscopically.
The amount of tissue sampled for histology may only represent a minority of the resected tissue, depending on the size of the specimen (6). It is possible that the reported closest resection margin may not be the real closest margin due to sampling error. This can partly explain why it is not a surprise to have local recurrence appearing at the same surgical field in the patients with a negative histology margin. Abraham had three local recurrences out of 21 resections with negative resection margin (1). Moreover, these local recurrences may also be examples of skip lesions of certain centimeters from the main tumors.
As tumor surgeons, we are more concerned with the local recurrence in the long run. The risk of the local recurrence may be predicted by the planned margin in the pre-operative plan. The wider the planned margin, it is more likely that the whole tumor, including the pseudopodia and satellites, is within the resected specimen. Unfortunately, even for the widest margin (curative), there is still few percent of local recurrence (3).
Soft tissue margin in the resection of pelvic and sacral tumors
Currently, the navigation-assisted surgery can only work on the bone cut. It cannot be applied to the soft tissue part of the pelvic and sacral tumor surgery. There is no way to navigate the soft tissue cut because of the flexibility and elasticity of the soft tissue. The intra-operative method of identifying the 3D anatomy of soft tissue is still far from routine clinical use.
In the paper, the authors (1) reported their 23 navigation-assisted surgeries in resection of pelvic and sacral tumors. There was no “margin involvement” by tumor in the bony margin. However, the soft tissue “margin involvement” was in 2 out of 23 surgeries. In 2013, Jeys et al. (8) showed a similar result. Soft tissue involvement was in 3 out of 31 patients. There was also no bone margin involvement. This reflected how unreliable navigation-assisted surgery might be in soft tissue margin in these resections.
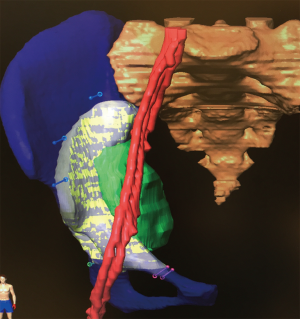
In our hospital, we use the navigation software to build up a 3D virtual image of both soft tissue and bony components of the pelvic and sacral tumors (Figure 1) in order to have better visualization of the relationship between various anatomical structures. These 3D soft tissue images are simply for the pre-operative mental planning stage of the resection. It is far from clinical use in execution of the pre-operative resection plan through the navigation system.
Studies in the literature
Sternheim et al. (9) in 2015 studied the accuracy and reproducibility of the navigated osteotomy in sawbones and cadaveric bones. The mean entry cut and exit cut for sawbones were 1.4±1 and 1.9±1.2 mm from the plan. It is significantly better than the non-navigated cut. For the cadaveric bone, they were 1.5±0.9 and 2.1±1.5 mm correspondingly. Cartiaux et al. (10), in the sawbone model study, found that 22% of “non-navigated cut” went intra-lesionally whereas no “navigated cut” went intra-lesionally.
Laitinen et al. (11) analyzed the oncological outcome of their patients with tumor resections involving posterior ilium and sacrum. Local recurrence rate was 22.2% in navigation-assistance whilst non-navigation assisted was 50%. Another report also by Jeys et al. in 2013 (8) had 13% of local recurrence in their primary bone tumour of pelvis and sacrum resection under computer navigation assistance. Their local recurrence rate before the era of navigation-assisted surgery was 27%.
Conclusions
The navigation-assisted resection can only improve the precision in the bone cut but it cannot compensate for any poorly designed cut in the pre-operative planning of surgical margin to achieve a negative histology margin. In addition, navigation assisted surgery can help in achieving negative margin in bone cut but not in the soft tissue. Readers should understand these points clearly before applying the technology to their patients.
Acknowledgements
We thank Ms. Carol Anne Higgins for her contribution in proofreading the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Abraham JA, Kenneally B, Amer K, et al. Can navigation-assisted surgery help achieve negative margins in resection of pelvic and sacral tumors? Clin Orthop Relat Res 2018;476:499-508. [Crossref] [PubMed]
- Cardona K, Movva S. Issues in the management of high-risk localized sarcomas. Curr Probl Cancer 2013;37:62-73. [Crossref] [PubMed]
- Kawaguchi N, Ahmed AR, Matsumoto S, et al. The concept of curative Margin in surgery for bone and soft tissue sarcoma. Clin Orthop Relat Res 2004.165-72. [Crossref] [PubMed]
- So TY, Lam YL, Mak KL. Computer-assisted navigation in bone tumor surgery: seamless workflow model and evolution of technique. Clin Orthop Relat Res 2010;468:2985-91. [Crossref] [PubMed]
- Enneking WF, Spanier SS, Goodman AA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res 1980.106-20. [PubMed]
- White LM, Wunder JS, Bell RS, et al. Histologic assessment of peritumoral edema in soft tissue sarcoma. Int J Radiat Oncol Biol Phys 2005;61:1439-45. [Crossref] [PubMed]
- Rubin BP, Antonescu CR, Gannon FH, et al. Protocol for the examination of specimens from patients with tumors of bone. Arch Pathol Lab Med 2010;134:e1-7. [PubMed]
- Jeys L, Matharu GS, Nandra RS, et al. Can computer navigation-assisted surgery reduce the risk of an intralesional margin and reduce the rate of local recurrence in patients with a tumour of the pelvis or sacrum? Bone Joint J 2013;95-B:1417-24. [Crossref] [PubMed]
- Sternheim A, Daly M, Qiu J, et al. Navigated pelvic osteotomy and tumor resection: a study assessing the accuracy and reproducibility of resection planes in Sawbones and cadavers. J Bone Joint Surg Am 2015;97:40-6. [Crossref] [PubMed]
- Cartiaux O, Banse X, Paul L, et al. Computer-assisted planning and navigation improves cutting accuracy during simulated bone tumor surgery of pelvis. Comput Aided Surg 2013;18:19-26. [Crossref] [PubMed]
- Laitinen MK, Parry MC, Albergo JI, et al. Is computer navigation when used in surgery of iliosacral pelvic bone tumours safer for the patient? Bone Joint J 2017;99-B:261-6. [Crossref] [PubMed]


