
Management of symptomatic sacral perineural cysts with microsurgery and a vascularized fasciocutaneous flap
Introduction
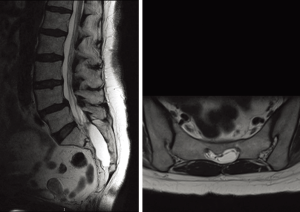
Perineural or Tarlov cysts were initially described in 1938 during cadaveric observations of terminal filum specimens (1-3). Their origin is largely unknown (3-6). According to the classification by Nabors et al., Tarlov cysts are considered type II meningeal cysts defined as “extradural meningeal cysts with spinal nerve roots” (7). Most are discovered incidentally on magnetic resonance imaging (MRI) scans and approximately 13% are considered symptomatic (3,6,8,9). The most common complaints attributed to them are low back pain, sciatica, perineal discomfort, pain or numbness and urinary/bowel problems (3,8,10-13).
There is no general agreement on the optimal management of symptomatic Tarlov cysts. Conservative or minimally invasive methods such as cyst aspiration and cerebrospinal fluid (CSF) diversion have provided conflicting results since symptomatic cyst recurrence and aseptic meningitis can occur (3,5,14-16). Surgical techniques have evolved over the years and include procedures such as laminectomy or laminotomy and decompression, cyst fenestration, cyst resection, cyst imbrication (wall fixed to neighboring structures), sacroplasty and removal of the proposed ball-valve mechanism (5,10-13,17-22).
In recent years, mobilization of muscle flaps to augment cyst closure, minimize cyst recurrence and wound related complications has been increasingly used during surgery (10,11,23). Here, we present our operative series of symptomatic Tarlov cysts with microsurgery and use of a vascularized fasciocutaneous flap.
Methods
Patients
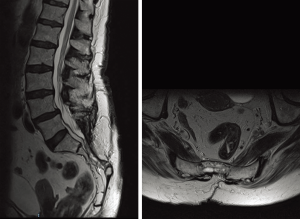
Patients surgically treated from October 2013 to November 2016 for symptomatic sacral perineural/Tarlov cysts were included. The cysts were identified on MRI (Figure 1). Patients considered candidates for surgery were typically primarily subjected to a diagnostic cyst puncture. A transient relief of symptoms followed by symptom relapse and associated with cyst recurrence on MRI led to the decision to proceed to surgery. Moreover, patients with persistent radicular symptoms, pelvic pain and sphincter problems (urine and bowel incontinence) refractive to conservative treatments including anti-nociceptive treatment or physical therapy were also considered candidates for surgery, given that complaints were unrelated to other processes such as degenerative spine disease, tumors, infections and traumatic lesions excluded by MRI and medical history.
Surgical technique
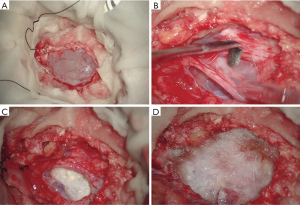
Patients were placed in prone position on a Wilson frame. A midline incision at the level corresponding to the cyst was undertaken. After opening the lumbosacral fascia, subperiosteal dissection of the surrounding muscles from the spinous processes and laminae was done. Next, a laminectomy at the level of interest (usually L5–S2) was performed to expose the cyst. The cyst was then opened and excised under the microscope. The area was inspected and the nerve roots were identified. Whenever possible, the nerve roots were released from the cyst wall and surrounding tissues using microneurosurgical techniques and neuromonitoring. When present, communication between the cyst and the subarachnoid space was identified and sealed with TachoSil packing and fibrin glue if neural elements were not included. When possible, the remaining cyst wall was sutured with a running non-absorbable suture but even in these cases, watertight closure could not be accomplished. Packing with Surgicel® (Ethicon, USA) and/or TachoSil® (Kaneda, Japan) followed and fibrin glue (Tisseel®, Baxter, USA) was placed over the cyst wall (Figure 2).
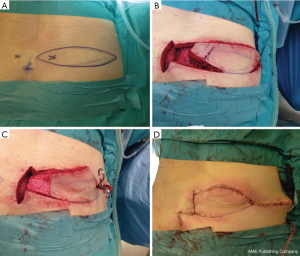
A plastic and reconstructive surgeon (MM) mobilized a local perforator fasciocutaneous flap to carefully cover the area after cyst evacuation. Specifically, a lumbar perforator was identified with a handheld Doppler lateral to the spinal column. The perforator was included in the flap once designed and marked pre-operatively. The flap was either designed as a broad-based V to Y fashion with one edge transposed into the space created by cyst opening/ excision or as a flap isolated on the specific perforator and rotated into the defect (named a propeller flap). The tissue positioned into the defect was de-epitheliased and anchored with 3.0 Monocryl sutures. A drain was placed in the donor-site area and the wound was closed in an ordinary three-layer fashion (Figure 3). In patients with large cysts, large dural defects and unsecure closure, a lumbar drain was also placed following surgery.
Postoperative care and follow-up
The wound drain was removed 24 hours postoperatively. The lumbar drain typically drained for 3–5 days, then it was clamped, the wound was inspected for CSF leakage and removed if no leakage or symptoms of intracranial hypotension were observed. Patients were followed-up by consecutive visits to the treating physicians where a detailed neurological examination was undertaken, and post-operative MRI scans. Possible relapse of symptoms, complications, reoperations and cyst recurrence were recorded.
Results
Baseline characteristics
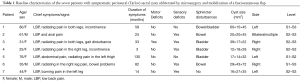
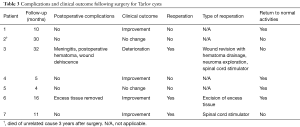
Seven patients were included (Table 1). The mean age was 50.3 years (range, 25–80 years) and the mean duration of symptoms was 49.3 months (range, 3–130 months). The major complaint was back pain, present in all patients. One patient exhibited decreased motor strength in the lower limbs. All but one patient presented with urine and/or bowel incontinence. All cysts had a maximum diameter ≥2 cm. No patient had a coexisting spina bifida.
Full table
Surgical results
Prior to the present surgery, all but one patient received surgical treatment either minimally invasive by cyst puncture or open surgery. Intraoperatively, a CSF fistula was identified in one patient and the communication was successfully sealed. In only two patients, the cyst wall was closed with a running suture. In the remaining patients, the cyst was packed with Surgicel and TachoSil and fibrin glue was also applied. A V-Y muscle flap was mobilized in five patients while a propeller flap was used in the remaining two. A wound drain was inserted in 4/7 patients and a lumbar drain in 5/7 patients (Table 2).

Full table
Postoperative course and complications
The follow-up period was 15.4 (range, 4–32) months. Symptoms improved in four patients, two patients experienced transient relief but symptoms later recurred while one patient had deteriorated clinically at long-term. One patient developed meningitis, postoperative hematoma and wound rupture and one was reoperated for removal of excess skin tissue. In two cases, a dorsal column stimulator was implanted due to persisting neurogenic pain. In half of the patients (2/4), urine incontinence improved. In addition, 4/7 patients returned to normal activities of daily life. Bowel disturbances did not improve. In all seven cases, a significant decrease in cyst size was noted on MRI (Table 3, Figure 4).
Full table
Discussion
In the current patient cohort of surgically treated sacral perineural (Tarlov) cysts, the use of microsurgical technique and a fasciocutaneous vascularized flap resulted in significant resolution of symptoms in more than half of the patients. Only one patient deteriorated following surgery. Half patients with urine incontinence experienced improved bladder control. Serious complications were rare and observed in only one patient. The use of a vascularized flap aided in the significant reduction in cyst wall size in all operated cases.
Lumbosacral Tarlov cysts are uncommon lesions, occurring in approximately 1–5% of the general population and located at the junction of the dorsal ganglion and the posterior nerve root between the endoneurium and the perineurium (5,12,15,24). Although still unclear, a valve-like mechanism between the subarachnoid space with the dura leading to CSF inflow is considered the most probable pathogenetic theory of their formation (5,7,25). Genetic factors may also contribute to their pathogenesis (4).
Even in symptomatic cysts, the indications to proceed to surgery have not been defined and most published reports describe a limited number of patients (6,12,13,18,20-22,25-27). The following surgical indications for symptomatic patients with Tarlov cysts were recently suggested: (I) multiple cysts on MRI; (II) symptoms related to the location of the cyst; (III) symptoms appeared in the past 6 months; and (IV) single, unilateral cyst over 1 cm in size (10). In particular, younger patients with single unilateral cysts with medically refractory symptoms have mostly benefited from surgery (10). We suggest that patients with progressive and/or refractory lumbosacral or radicular pain, urogenital pain and persistent urine incontinence attributed to the presence of the cyst are appropriate candidates for surgery (5,10,26).
Numerous more or less invasive surgical techniques have been proposed for the treatment of Tarlov cysts (5,8,10-12,17-21,28). The overall goal is alleviation of symptoms with radiological evidence of decrease in cyst size and prevention of further sacral bone erosion. The results have been conflicting with the best clinical outcomes observed following the use of direct microsurgical approaches (5,8,10). From the surgical perspective, neural decompression is important, and equally important is a meticulous closure and obliteration of the cyst wall and the overlying layers with the aim to avoid postoperative cyst and symptom recurrence and CSF leakage. Outcome following surgery for symptomatic Tarlov cysts has been recently reviewed. Of the 646 patients that were included in the analysis, 32% reported complete alleviation of symptoms, 50% partial resolution and 16% no improvement or clinical deterioration. Large cysts were not associated with complete resolution of symptoms (8). In the current series, cyst size did not influence the postoperative results.
Recent reports have used paraspinal muscle flaps to assist in wound closure and prevent cyst recurrence (10,23). In 35 patients with symptomatic Tarlov cysts, after microsurgical opening of the cyst and repair of the cyst wall, a multifidus muscle pedicle flap was mobilized to fill the cyst cavity. The muscle flaps were rotated into the sacral cavity and occasionally sutured to the ligamentum flavum. Using this technique, 93% reported improvement at some time postoperatively. Nevertheless, symptoms recurred in half patients, and three patients required reoperation due to pseudomeningocele. It should be noted, however, that uniform muscle atrophy was frequently seen on postoperative images attributed to impaired innervation caused by the functional reconstruction of the muscles (11).
The use of vascularized fasciocutaneous flaps, as in the present study, reduces the risk of progressive muscle atrophy and provides better conditions for a long-lasting obliteration of the cyst cavity. This was also evident in the current case series. As such, fasciocutaneous flaps may offer better chances for an effective surgical treatment of Tarlov cysts in a long-term perspective.
Conclusions
Cyst fenestration and the use of a vascularized fasciocutaneous flap enabled the obliteration of the cyst in all patients with Tarlov cysts. Although incomplete remission of symptoms or persistent complaints were not infrequent after surgery, the overall clinical outcome was favorable. Careful patient selection remains the most important factor determining outcome following surgery for sacral perineural cysts. Although we suggest initially a cyst puncture and in case of symptom regression, cyst excision and mobilization of a fasciocutaneous flap over the cyst wall, larger series are justified to clarify the long-term effects of this combined surgical approach in patients with symptomatic lesions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the regional ethics committee of Uppsala University Hospital (Spinal Research No. 400/2012). All procedures performed in studies involving human participants are in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Tarlov IM. Perineural cysts of the spinal nerve roots. Arch Neurol Psychiatr 1938;40:1067-74. [Crossref]
- Tarlov IM. Spinal perineurial and meningeal cysts. J Neurol Neurosurg Psychiatry 1970;33:833-43. [Crossref] [PubMed]
- Acosta FL Jr, Quinones-Hinojosa A, Schmidt MH, et al. Diagnosis and management of sacral Tarlov cysts. Case report and review of the literature. Neurosurg Focus 2003;15. [Crossref] [PubMed]
- Park HJ, Kim IS, Lee SW, et al. Two cases of symptomatic perineural cysts (tarlov cysts) in one family: a case report. J Korean Neurosurg Soc 2008;44:174-7. [Crossref] [PubMed]
- Lucantoni C, Than KD, Wang AC, et al. Tarlov cysts: a controversial lesion of the sacral spine. Neurosurg Focus 2011;31. [Crossref] [PubMed]
- Voyadzis JM, Bhargava P, Henderson FC. Tarlov cysts: a study of 10 cases with review of the literature. J Neurosurg 2001;95:25-32. [PubMed]
- Nabors MW, Pait TG, Byrd EB, et al. Updated assessment and current classification of spinal meningeal cysts. J Neurosurg 1988;68:366-77. [Crossref] [PubMed]
- Dowsett LE, Clement F, Coward S, et al. Effectiveness of Surgical Treatment for Tarlov Cysts: A Systematic Review of Published Literature. Clin Spine Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Langdown AJ, Grundy JR, Birch NC. The clinical relevance of Tarlov cysts. J Spinal Disord Tech 2005;18:29-33. [Crossref] [PubMed]
- Burke JF, Thawani JP, Berger I, et al. Microsurgical treatment of sacral perineural (Tarlov) cysts: case series and review of the literature. J Neurosurg Spine 2016;24:700-7. [Crossref] [PubMed]
- Potts MB, McGrath MH, Chin CT, et al. Microsurgical Fenestration and Paraspinal Muscle Pedicle Flaps for the Treatment of Symptomatic Sacral Tarlov Cysts. World Neurosurg 2016;86:233-42. [Crossref] [PubMed]
- Guo D, Shu K, Chen R, et al. Microsurgical treatment of symptomatic sacral perineurial cysts. Neurosurgery 2007;60:1059-65; discussion 1065-6. [Crossref] [PubMed]
- Weigel R, Polemikos M, Uksul N, et al. Tarlov cysts: long-term follow-up after microsurgical inverted plication and sacroplasty. Eur Spine J 2016;25:3403-10. [Crossref] [PubMed]
- Bartels RH, van Overbeeke JJ. Lumbar cerebrospinal fluid drainage for symptomatic sacral nerve root cysts: an adjuvant diagnostic procedure and/or alternative treatment? Technical case report. Neurosurgery 1997;40:861-4; discussion 864-5. [Crossref] [PubMed]
- Paulsen RD, Call GA, Murtagh FR. Prevalence and percutaneous drainage of cysts of the sacral nerve root sheath (Tarlov cysts). AJNR Am J Neuroradiol 1994;15:293-7; discussion 298-9. [PubMed]
- Patel MR, Louie W, Rachlin J. Percutaneous fibrin glue therapy of meningeal cysts of the sacral spine. AJR Am J Roentgenol 1997;168:367-70. [Crossref] [PubMed]
- Rodziewicz GS, Kaufman B, Spetzler RF. Diagnosis of sacral perineural cysts by nuclear magnetic resonance. Surg Neurol 1984;22:50-2. [Crossref] [PubMed]
- Caspar W, Papavero L, Nabhan A, et al. Microsurgical excision of symptomatic sacral perineurial cysts: a study of 15 cases. Surg Neurol 2003;59:101-5; discussion 105-6. [Crossref] [PubMed]
- Fogel GR, Cunningham PY 3rd, Esses SI. Surgical evaluation and management of symptomatic lumbosacral meningeal cysts. Am J Orthop (Belle Mead NJ) 2004;33:278-82. [PubMed]
- Mummaneni PV, Pitts LH, McCormack BM, et al. Microsurgical treatment of symptomatic sacral Tarlov cysts. Neurosurgery 2000;47:74-8; discussion 78-9. [PubMed]
- Cantore G, Bistazzoni S, Esposito V, et al. Sacral Tarlov cyst: surgical treatment by clipping. World Neurosurg 2013;79:381-9. [Crossref] [PubMed]
- Tanaka M, Nakahara S, Ito Y, et al. Surgical results of sacral perineural (Tarlov) cysts. Acta Med Okayama 2006;60:65-70. [PubMed]
- Sonntag VK. Flap the Cyst. World Neurosurg 2016;87:659-60. [Crossref] [PubMed]
- Park HJ, Jeon YH, Rho MH, et al. Incidental findings of the lumbar spine at MRI during herniated intervertebral disk disease evaluation. AJR Am J Roentgenol 2011;196:1151-5. [Crossref] [PubMed]
- Neulen A, Kantelhardt SR, Pilgram-Pastor SM, et al. Microsurgical fenestration of perineural cysts to the thecal sac at the level of the distal dural sleeve. Acta Neurochir (Wien) 2011;153:1427-34; discussion 1434. [Crossref] [PubMed]
- Kunz U, Mauer UM, Waldbaur H. Lumbosacral extradural arachnoid cysts: diagnostic and indication for surgery. Eur Spine J 1999;8:218-22. [Crossref] [PubMed]
- Xu J, Sun Y, Huang X, et al. Management of symptomatic sacral perineural cysts. PLoS One 2012;7. [Crossref] [PubMed]
- Asamoto S, Fukui Y, Nishiyama M, et al. Diagnosis and surgical strategy for sacral meningeal cysts with check-valve mechanism: technical note. Acta Neurochir (Wien) 2013;155:309-13. [Crossref] [PubMed]


