
The evolution of partial undercutting facetectomy in the treatment of lumbar spinal stenosis
Lumbar spinal stenosis has been a common diagnosis in spinal pathology since it was originally described by Verbiest in 1954 (1). Previously, Putti in 1927, recognised that sciatica was due to an anomaly of facets causing secondary arthritis, “a neuralgia caused by pathological conditions of the intervertebral foramina and especially of the intervertebral articulations” (2). Lumbar nerve root entrapment was described in the lateral recess of the spinal canal of normal dimensions (3,4) and in those with a “shallow recess” and reduced dorso-ventral diameter (5). In both cases, entrapment by facet joints was the offending lesion. Macnab highlighted discal pathology as a more common cause of back pain and sciatica in younger patients as opposed to arthritis of the facet joints in older patients (6). The onset of axial tomography around this time allowed cross sectional studies of the lumbar spine thus overcoming the limitations of two-dimensional imaging such as myelography (7). Stenosis was thus described and studied in greater detail (8).
Lumbar decompression is the most common spinal operation in patients over 60 years of age. While decompression was considered an appropriate technique in the treatment of spinal stenosis, the study by Getty et al. in 1981 was significant in describing the technique of stability preservation in surgical decompression (9). They assessed 78 treated patients with predominant leg symptoms and degenerative change in the posterior facet joints. Decompression was achieved by a partial undercutting facetectomy, achieving a satisfactory outcome in 85% of cases. Patients were advised that the recommended treatment had a “variable effect on backache”—an outcome of decompressive surgery that has since been extensively reported but has not changed. Similarly, Sanderson et al. reported on 57 patients who underwent partial undercutting laminectomy with 88% mild or no leg pain at mean 8.4 years (10). More recent data for both decompression and combined decompression-fusion procedures in lumbar stenosis from the Swedish registry have shown mean EuroQol-visual analogue scales (EQ-VAS) increases from 50.2 to 65.2, mean VAS decreases from 64 to 34, 77% improvement of leg pain and patients able to walk 500 m increased from 27% to 65%, at 1 year. The difference between the fusion and non-fusion patients with back pain on the VAS was a mean of only 0.3 (11). Again, this is also reflective of the back-pain component of spinal stenosis and of a technique that has potential for improvement.
In the original technique described by Getty et al., a laminotomy fenestration is performed at the level of the relevant nerve root. The width of the pars interarticularis is outlined and the bony dimple on its medial aspect is located and removed, allowing visualisation of the root. It is safe to notch the medial aspect of inferior lamina with a 45˚ Codman rongeur to access the canal. An osteotome can then be applied along the inferior edge of the lamina using the apex of the notch as a start point. A curved end osteotome (not unlike a large gouge) can make arched cuts whose propagated cracks exit safely along the cut surface.
The direction of the root canal can be defined with gentle probing, then decompressed using a 10 mm osteotome which is advanced in an oblique direction, initially in the line of the nerve root. A Mac Donald dissector can be interposed between the root and the facet to provide additional safety. The osteotome is advanced with rapid light blows of the mallet to reduce further risk of sudden uncontrolled advance. The use of a Kerrison’s rongeur or a similar instrument in this narrow space is considered dangerous.
The initial osteotomy is obliquely through the inferior articular process of the upper vertebra at the level of the decompression. When the articular surface of the superior articular process of the lower vertebra is reached, the osteotome is twisted to free the osteotomised fragment which is eased out with a rongeur. The osteotome is then advanced through the full length of the superior articular process of the lower vertebra which is causing compression. The lateral attachments of the ligamentum flavum are removed with this portion of the bone, sharp dissection being used when it is necessary. The removal of more bone from the lamina of the uppermost vertebra may be necessary to give access to the uppermost part of the facet joints. When hypertrophy and subluxation of the facets hide the nerve root, osteotomy is performed in the same way, including around the pedicle. Provided that the root is identified where it arises from the dural sac and the described precautions are taken, the root will not be damaged. A nerve root may be trapped by bone at two levels, and it is sometimes necessary to undercut the facet joints at both levels, which may require hemi-laminectomy. Careful search and clearance is required for a sequestered disc.
At the time, Getty et al. warned against the use of the Kerrison rongeur. In cases where the central canal is tight there is very little space to insert the rongeur and the flavum may be adherent to the dura. Blind cutting of the ligamentum flavum and the lamina by the rongeur risks a dural tear. As the dura recoils back when the rongeur is removed after excising a fragment of lamina and/or ligamentum flavum the sharp ends of the bone can puncture the dura. Use of the Lambotte osteotome is highly user dependent, particularly regarding its potential for plunging or propagating a tangential linear crack. This is especially relevant in revision cases where scarring distorts the normal anatomy and where there is already an increased potential for causing instability.
Surgical strategies for the decompression of lumbar spinal stenosis have evolved to include minimally invasive techniques providing for adequate and safe decompression while reducing perioperative morbidity. Improved illumination and visualisation with microscopic or micro-endoscopic minimally invasive decompression have shown reduced length of stay, minimal requirements for narcotic pain medications, and a low rate of readmission and complications (12). A recent metanalysis has shown that minimally invasive surgery has a 3.3% secondary fusion rate compared with 12.8% with open surgery and total reoperation rates of 5.8% compared with 16.3% (13). These techniques have also shown the ability to angulate the access port, while preserving the interspinous ligaments, aiming posterior to the central dura to focus on the contralateral side, providing the ability to decompress the contralateral neural foramen. Attempts to preserve the midline structures through spinous process splitting approaches have shown some early reductions in pain and reduced postoperative muscle atrophy but without any difference in outcomes at 1 year (14,15).
The most popular resectional tool is the high-speed burr. With its acorn shaped end, the burr is versatile at removing bone and particularly decorticating laminae for fusion. It is most effective at removing the dorsal cortex and cancellous core of the inferior lamina at the superior level of the decompression, which can then be followed by a kerrison rongeur (16). While frequently used for decompression, the rotary macro-motion of a drill’s burr around the dura or neural structures is limited by its potential for spinning off the target area. Like a micro-saw, the potential for lateral tip straying is high and the teeth can grab adjacent soft tissue or cottonoids. While poorly described in the literature, it is known that spin off can occur with the burr into the dura and associated nerve root entanglement. This may be increasing difficult to control in patients with harder bone (for example, Afro-Caribbean) or those on anti-resorptive therapies. If burrs are to be used, an electric powered burr may be safer than air. When depowering the electric burr, it stops rotating instantly, whereas some traditional air powered burrs can continue spinning after depower.
The ultrasonic bone cutter has promoted more defined and precise bone cuts. Its safety profile is superior as it does not have a spinning motion but an oscillatory one. It has a relative selectivity for bone ablation where bone must be cut adjacent to dura and neural structures. The frequency is typically above 20 kHz, exceeding the audible frequency range. This ultrasonic energy is transferred from a blade to tissue molecules, which begin to vibrate in response and are ablated by frequencies in the low ultrasonic range in dense tissues such as bone. Soft tissue structures, by contrast (such as ligamentum flavum, posterior longitudinal ligament, and dura) can bend, deform, move away, and vibrate upon contact with the blade, thus dampening the energy transfer and protecting the tissue from destruction. The tip is irrigated which requires constant directed suctioning. The technique involves lateral movement with minimal axial pressure through the outer cortex, more liberal progression through the cancellous mid-portion, then controlled short cyclical sweeps to penetrate the inner cortex. Once through this cortex, the tactile feedback allows the operator to develop the opening from the edge by withdrawing the blade, momentarily stopping the ultrasonic action, palpating the inner cortex with the blade and then resuming cutting.
With traditional tools, the zones at most risk of an incidental durotomy include the cephalad and caudal laminar margins, the medial aspect of the facet joint and potentially on approaching the disc if this were to warrant removal. Plunging the ultrasonic bone cutter as with any surgical tool risks cutting the dura. Repetitive cutting over the dura may also cut it—from excessive heat and a thermal lesion. Adhesions from an epidural scar or with ossification of the posterior longitudinal ligament disallow the dura from moving away from the inner cortex and are thus at risk. Even after cutting the inner cortex uneventfully, removing the bone may prompt a dural tear. Thus the adherent dura should be dissected off first with an forward-angled curette, then the bone removed in sequential slices until an adequate decompression has been achieved. Cutting slices are a safe approach to identifying how much bone warrants removal.
The laminotomy cuts for the ultrasonic bone cutter rely on the same principles as described by Getty et al. (9)—fenestration of the inferior lamina at the spino-laminar junction, resection of the medial pars dimple, oblique osteotomy through the inferior articular process then the superior articular facet (undercutting) (Figure 1). After making the approach, the spinous process can be partially removed with a rongeur or split in a T shape fashion down to the intact base. Reduced bone bleeding has been observed with the ultrasonic bone cutter (17). This is relevant for spinous splitting decompression approaches, where haematoma formation over the decompression site has traditionally been a problem—thus lending its use to minimally invasive surgery.
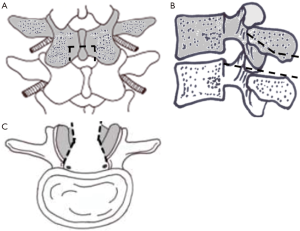
A central transverse cut through the inferior half of the spinous process is advised, as opposed to removing the entire spinous process—thus only sacrificing one interspinous segment. This is followed by sagittal longitudinal cuts through the inferior articular processes thus de-roofing the spinal canal. The opening does not need to be more than 1.5 cm on each side for a single level, shorter in the proximal lumbar spine. The transverse cuts are particularly effective for central canal stenosis, where the decompression can be extended cephalad and caudally. It is important not to remove the ligamentum flavum too early as it protects the dura. The root may not be visible if it is displaced anteriorly because of subluxation and facet overgrowth. Oblique cuts can be made along each of the 4 walls of this rectangular opening to create an inverted funnel effect. The undercutting partial facetectomy is performed on each side and a ridge of bone is best teased off slowly and progressively with a pituitary rongeur. The anterior aspect of the pars interarticularis can be thinned but at least 5 mm width should be preserved. The sharp edge of the resected fragment at its deepest aspect may tear the dura so it should be twisted away from the dura. For foraminal stenosis with a severely collapsed disc space and a bony spur, decompression without touching off the exiting nerve is difficult. Safe removal of bone around the root at the level of the pedicle is achievable with repeat cuts. Placing two markers—such as a Watson-Cheyne dissector at either end of the dissection is useful for fluoroscopic identification of an adequate decompression. The use of the ultrasonic bone cutter has also been described with the use of a microscope (18).
Combining the merits of modern techniques to provide superior short- and long-term outcomes is the ultimate goal in lumbar decompression surgery. Preservation of spinal stability is pivotal to the outcomes of lumbar decompression surgery, as espoused by Getty et al. (9). Conventional techniques through a minimally traumatic surgical corridor, optimal visualisation, safe well-defined osteotomy lines and adequate decompression of stenotic pathology will ultimately build on achieving improved clinical outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Verbiest H. A radicular syndrome from developmental narrowing of the lumbar vertebral canal. J Bone Joint Surg Br 1954;36-B:230-7. [Crossref] [PubMed]
- Putti V. New conceptions in the pathogenesis of sciatic pain. Lancet 1927;ii:53-60. [Crossref]
- Epstein JA, Epstein BS, Lavine LS, et al. Lumbar nerve root compression at the intervertebral foramina caused by arthritis of the posterior facets. J Neurosurg 1973;39:362-9. [Crossref] [PubMed]
- Choudhury AR, Taylor JC. Occult lumbar spinal stenosis. J Neurol Neurosurg Psychiatry 1977;40:506-10. [Crossref] [PubMed]
- Schatzker J, Pennal GF. Spinal stenosis, a cause of cauda equina compression. J Bone Joint Surg Br 1968;50:606-18. [Crossref] [PubMed]
- Macnab I. Backache. Baltimore: Williams & Wilkins, 1977.
- Gargano FP. Transverse axial tomography of the lumbar spine. In: Rothman RH, Simeone FA. Editors. The spine. Philadelphia: WB Saunders Company, 1975;469-75.
- Postacchini F, Pezzeri G, Montanaro A, et al. Computerised tomography in lumbar stenosis. A preliminary report. J Bone Joint Surg Br 1980;62-B:78-82. [Crossref] [PubMed]
- Getty CJ, Johnson JR, Kirwan EO, et al. Partial undercutting facetectomy for bony entrapment of the lumbar nerve root. J Bone Joint Surg Br 1981;63-B:330-5. [Crossref] [PubMed]
- Sanderson PL, Getty CJ. Long-term Results of Partial Undercutting Facetectomy for Lumbar Lateral Recess Stenosis. Spine (Phila Pa 1976) 1996;21:1352-6. [Crossref] [PubMed]
- Jansson KA, Németh G, Granath F, et al. Health-related quality of life (EQ-5D) before and one year after surgery for lumbar spinal stenosis. J Bone Joint Surg Br 2009;91:210-6. [Crossref] [PubMed]
- Podichetty VK, Spears J, Isaacs RE, et al. Complications associated with minimally invasive decompression for lumbar spinal stenosis. J Spinal Disord Tech 2006;19:161-6. [Crossref] [PubMed]
- Schöller K, Alimi M, Cong GT, et al. Lumbar Spinal Stenosis Associated With Degenerative Lumbar Spondylolisthesis: A Systematic Review and Meta-analysis of Secondary Fusion Rates Following Open vs Minimally Invasive Decompression. Neurosurgery 2017;80:355-67. [Crossref] [PubMed]
- Watanabe K, Matsumoto M, Ikegami T, et al. Reduced postoperative wound pain after lumbar spinous process-splitting laminectomy for lumbar canal stenosis: a randomized controlled study. J Neurosurg Spine 2011;14:51-8. [Crossref] [PubMed]
- Rajasekaran S, Thomas A, Kanna RM, et al. Lumbar spinous process splitting decompression provides equivalent outcomes to conventional midline decompression in degenerative lumbar canal stenosis: a prospective, randomized controlled study of 51 patients. Spine (Phila Pa 1976) 2013;38:1737-43. [Crossref] [PubMed]
- Kleeman TJ, Hiscoe AC, Berg EE. Patient outcomes after minimally destabilizing lumbar stenosis decompression: the "Port-Hole" technique. Spine (Phila Pa 1976) 2000;25:865-70. [Crossref] [PubMed]
- Hu X, Ohnmeiss DD, Lieberman IH. Use of an ultrasonic osteotome device in spine surgery: experience from the first 128 patients. Eur Spine J 2013;22:2845-9. [Crossref] [PubMed]
- Ito K, Ishizaka S, Sasaki T, et al. Safe and minimally invasive laminoplastic laminotomy using an ultrasonic bone curette for spinal surgery. Surg Neurol 2009;72:470-5; discussion 475. [Crossref] [PubMed]


