
Biomechanical evaluation of interbody fixation with secondary augmentation: lateral lumbar interbody fusion versus posterior lumbar interbody fusion
Introduction
Interbody hardware is used to restore the intervertebral space, reconstituting the load-bearing elements of the lumbar spine to provide stability and enhance fusion potential. The principal tenets of interbody fusion include placement of an interbody graft under direct compression, thereby restoring normal anatomical disc height, indirectly decompressing neural foramina, preserving posterior elements, and restricting motion across fusion segments (1). This frequently requires that interbody fusion be supplemented with dorsal fixation to achieve optimal construct rigidity.
Multiple approaches exist that provide several anatomical trajectories, each with potential trade-offs determined by the extent of resection of local supportive structures and graft size. The posterior lumbar interbody fusion (PLIF) and lateral lumbar interbody fusion (LLIF) procedures use different anatomical approaches and different interbody implants to attain lumbar arthrodesis. LLIF is a method of minimally invasive lumbar interbody fusion performed with a comprehensive set of instruments.
LLIF differs from PLIF in terms of its lateral approach and the structural characteristics of the interbody implant. In LLIF, the lateral transpsoas approach allows for preservation of back muscles, anterior and posterior longitudinal ligaments, and facet joints. LLIF has been shown to result in decreased tissue dissection and decreased operative time, as well as reduced postoperative pain (2). The higher profile and bilateral epiphyseal position of the interbody implant used in the LLIF approach provides strong support for disc height restoration and indirect neural foraminal decompression, and for improved correction of sagittal and coronal plane imbalance (3,4). However, the biomechanics of the interbody fusion construct used in the LLIF approach have not been rigorously compared with those of the PLIF approach in the presence of secondary augmentation.
Our objective was to directly compare the biomechanical stabilizing effect of the interbody fusion constructs used in the LLIF and PLIF approaches, both supplemented with secondary augmentation using pedicle screw-rod fixation, in flexion-extension, lateral bending, and axial rotation. Rotational range of motion (ROM) was used as the standard metric of comparison.
Methods
We hypothesized that the LLIF approach would provide greater stability than the PLIF approach with secondary augmentation.
Specimen preparation
Twenty-one human cadaveric L2–L5 specimens were studied. The mean ± SD age of the specimens was 55.2±13.5 years (range, 21–73 years); there were 11 male and 10 female cadaveric specimens. Neither institutional review board approval nor consent was deemed necessary due to the cadaveric nature of the study. By screening the medical records of the suppliers of cadaveric materials and plain film radiographs and by directly inspecting the specimens, we ensured that no specimen had any obvious pathology that might affect biomechanics, especially metastatic disease, osteophytes, disc narrowing, or joint arthrosis. Dual-energy X-ray absorptiometry scans to assess bone mineral density (BMD) were performed on the L4 vertebra of each specimen with a resulting mean of 0.797±0.187 g/cm2. Analysis of variance (ANOVA) was used to compare the mean age and BMD values among the three groups of specimens studied and showed no significant differences (mean age, P=0.38; mean BMD, P=0.37).
Specimens were wrapped in plastic bags and stored at −20 °C until tested. The specimens were thawed in a bath of normal saline at 30 °C and carefully cleaned of muscle tissue while all the ligaments, the joint capsules, and the discs were kept intact. For testing, the exposed endplate and facet articulations of L5 were reinforced with household wood screws and the screwheads and part of the vertebral body were embedded in a cylindrical metal fixture using fast-curing resin (Smooth-Cast; Smooth-On, Inc.), and attached to the base of the testing apparatus. The L2 vertebra was similarly embedded in a cylindrical metal fixture for pure moment load application.
Testing conditions
Specimens were divided into three groups with similar age (P=0.12) and bone quality (P=0.37), then tested in four conditions: (I) intact and (II-IV) instrumented at L3–L4, as follows:
(II) Interbody + bilateral pedicle screws (BPS) using the LLIF approach (referred to as the LLIF construct; n=7);
(III) Bilateral interbody + BPS using the PLIF approach (referred to as the PLIF construct; n=7);
(IV) No lumbar interbody fusion (LIF) + BPS (referred to as the no-LIF construct; n=7).
The interbody fusion constructs used in the LLIF approach consisted of unilaterally placed polyether ether ketone (PEEK) interbody implants (CLYDESDALE Spinal System; Medtronic, Inc.), whereas the constructs in the PLIF approach used bilaterally placed CAPSTONE PEEK Spinal System implants (Medtronic, Inc.).
All the constructs were supplemented with BPS (CD HORIZON SOLERA Spinal System; Medtronic, Inc.). The pedicle screw (6.5 mm × 45–55 mm) that was used was a multiaxial, top-loading, rigidly locking system, with a cobalt chrome screwhead and a titanium alloy (Ti-6Al-4V) shaft. A 4.75-mm diameter cobalt chrome rod was used. The interbody implants used in the LLIF and PLIF procedures varied to accommodate specific specimen anatomy height (PLIF, 10 mm; LLIF, 10–14 mm), width (PLIF, 10 mm; LLIF, 18 mm), and length (PLIF, 22–32 mm; LLIF, 50–60 mm). All implants were placed according to the manufacturer’s recommendations using standard surgical techniques and instrumentation.
The LLIF surgical technique involved annulotomy and thorough discectomy, whereas the PLIF involved annulus incision to remove the disc and prepare the endplates. The appropriately sized PEEK interbody implants were inserted unilaterally in the LLIF construct and bilaterally in the PLIF construct. The interbody insertion was followed by supplemental posterior fixation using BPS, with anteroposterior fluoroscopy used to verify the correct screw trajectory.
Biomechanical testing
In all conditions tested, specimens were studied using standard pure moment flexibility tests. For these tests, an apparatus was used in which a system of cables and pulleys imparted nondestructive, non-constraining torques in conjunction with a standard servohydraulic test system (MTS Systems Corp.), as we have described previously (5,6). This type of loading is distributed evenly to each motion segment, regardless of the distance from the point of loading (7,8). Maximum loads of 7.5 Nm were applied about the appropriate anatomical axes to induce the three different types of motion: flexion-extension, lateral bending, and axial rotation. Three preconditioning cycles were applied at 7.5 Nm for 60 seconds to allow for creep in each loading direction to ensure appropriate settling at the hardware-bone interface and to improve reproducibility of the results. During data collection, load was applied quasistatically in 1.5-Nm increments, with each incremental load held for 45 seconds to a maximum of 7.5 Nm (6).
Three-dimensional specimen motion in response to the applied loads during flexibility tests was determined automatically at 2 Hz using the Optotrak 3020 system (Northern Digital, Inc.). This system stereophotogrammetrically measures the three-dimensional displacement of infrared-emitting markers rigidly attached in a non-collinear arrangement to each vertebra. Custom software converts the marker coordinates to angles about each of the anatomical axes in terms of the motion segment’s own coordinate system (9). Spinal angles were calculated using a technique that provides the most appropriate results for describing three-dimensional spinal motion (10). When specimens were instrumented within each construct, fluoroscopy was used to ensure correct positioning of the hardware.
Three parameters were generated from the quasistatic load deformation data: angular ROM, zone of ligamentous laxity or lax zone (LZ), and zone of ligamentous stretching or stiff zone (SZ). The LZ and SZ are components of the ROM and represent the low-stiffness and high-stiffness portions of the typically biphasic load-deformation curve, respectively (10,11). The LZ is similar to Panjabi’s neutral zone but is more reproducible and refers to the zone in which there is minimal ligamentous resistance, whereas the neutral zone is the zone in which there is only frictional joint resistance (7,8). Larger values of LZ, SZ, or ROM indicate greater instability.
Analysis
The location at which LZ crossed to SZ was calculated by extrapolating the load deformation slope at data points corresponding to 4.5 Nm, 6.0 Nm, and 7.5 Nm to zero load using the method of least squares. Data were normalized such that each specimen served as its own control. Normalized values of LZ, SZ, and ROM were statistically analyzed using a one-way ANOVA, followed by Holm-Šidák tests, to determine whether outcome measures were significantly different among the various conditions of instrumentation. The level for statistical significance was set at P<0.05.
Results
No fractures were observed in specimens. No screws or rods demonstrated signs of fracture, loosening, or breakage in all conditions tested.
Instrumented constructs versus intact
Compared to the intact condition, all three configurations of instrumentation greatly decreased the mobility in all directions of motion (Table 1). The mean reductions in mobility with LLIF + BPS were 91% in flexion (P<0.001), 82% in extension (P<0.001), 81% in lateral bending (P<0.001), and 71% in axial rotation (P<0.001). The corresponding reductions with PLIF + BPS were 86% flexion (P<0.001), 80% extension (P<0.001), 80% lateral bending (P<0.001), and 60% axial rotation (P<0.005). For no-LIF + BPS compared to intact, mobility was decreased by 77% in flexion (P<0.001), 67% in extension (P<0.001), 73% in lateral bending (P<0.001), and 44% in axial rotation (P<0.002).
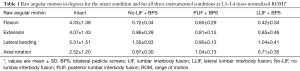
Full table
LLIF construct versus no-LIF construct
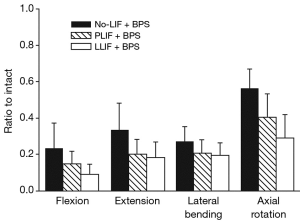
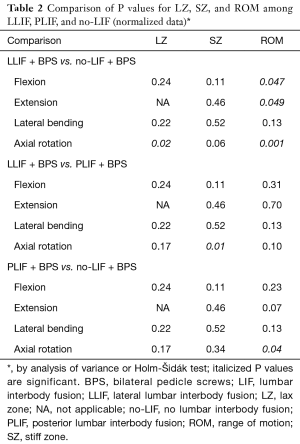
On the basis of our analysis of normalized values of motion (Figure 1, Table 2), LLIF + BPS was significantly more stable than no-LIF + BPS during flexion (P=0.047), extension (P=0.049), and axial rotation (P=0.001), but not during lateral bending (P=0.13). There were no significant differences between LLIF + BPS and no-LIF + BPS in terms of normalized SZ (P>0.06). The normalized LZ with LLIF + BPS was significantly less than with no-LIF + BPS during axial rotation (P=0.02), with no significant differences in flexion and lateral bending (P≥0.22).
Full table
PLIF construct versus no-LIF construct
The PLIF construct was significantly more stable than the no-LIF construct during axial rotation (P=0.04), with no significant differences in flexion (P=0.23), extension (P=0.07), and lateral bending (P=0.13). There were no significant differences between PLIF + BPS and no-LIF + BPS in LZ or SZ data (P≥0.11).
LLIF construct versus PLIF construct
The PLIF + BPS construct allowed similar normalized ROM to LLIF + BPS during flexion (P=0.31), extension (P=0.70), lateral bending (P=0.13), and axial rotation (P=0.10) (Figure 1). Normalized values of LZ and SZ were comparable (P≥0.11) for LLIF and PLIF constructs, with the exception of significantly larger SZ ROM in axial rotation in the PLIF construct (P=0.01).
Discussion
Biomechanical studies have demonstrated the benefit of larger profile interbody implants in interbody fusion (12). Lateral approaches [e.g., LLIF, extreme lateral interbody fusion (XLIF)] enable insertion of a larger interbody device, without sacrificing posterior elements and with theoretically improved stability compared to that of smaller interbody implants [e.g., PLIF, transforaminal interbody fusion (TLIF)] inserted via dorsal approaches (4). Studies have shown that stand-alone fusion constructs are not sufficiently stable without supplemental fixation, with many authors recommending additional posterior fixation to enhance stability (13-16). Although comparisons of stability of stand-alone constructs have been made between PLIF and LLIF (1,17), no prior studies have compared their relative stability when augmented with BPS. The purpose of the present study was to compare the biomechanical stability of interbody fusion constructs using the LLIF + BPS and PLIF + BPS approaches in the presence of supplemental dorsal fixation.
Our study demonstrates a similar biomechanical profile for the LLIF + BPS construct in providing immediate post-implantation stability relative to the PLIF + BPS construct, with somewhat greater effectiveness in limiting axial rotation with LLIF + BPS. Relative to the no-LIF + BPS construct, the LLIF + BPS construct demonstrated consistently smaller ROM in all modes of testing except lateral bending; comparatively, the PLIF + BPS construct demonstrated smaller ROM in axial rotation, with no differences in flexion, extension, or lateral bending.
Prior studies have shown the stabilizing benefit of supplemental fixation across all interbody devices (13-16). Laws et al. (18) demonstrated decreased ROM in flexion-extension and lateral bending, with increased stiffness approaching 350% in flexion and 220% in extension with the use of BPS along with an interbody implant in the LLIF approach. Fogel et al. (19) reported significant reductions in ROM in all loading modes with pedicle screw fixation after lateral interbody placement. Similarly, a comparative in vitro cadaveric study found that bilateral pedicle screw fixation dramatically improved stiffness in both the TLIF and the PLIF constructs; however, the PLIF construct demonstrated greater reductions, particularly in lateral bending. Despite the prevalence of pedicle screw fixation, a recent report by our group described comparable biomechanical stiffness of cortical screws and pedicle screws in both TLIF and LLIF constructs (6).
The stabilizing effect of posterior fixation may reduce biomechanical differences between interbody implants. Laws et al. (18) showed equivalent improvement in segmental stability, both with and without supplemental fixation, using the LLIF and the anterior lumbar interbody fusion (ALIF) approaches. Although the fusion construct had somewhat greater reduction in ROM with the LLIF than with the ALIF approach in all modes of testing without supplemental fixation, these results were not statistically significant. In comparison, Cappuccino et al. (1) reported minimal differences in biomechanical stability between the interbody fusion construct using the XLIF, ALIF, and TLIF constructs with supplemental bilateral posterior fixation. However, Pimenta et al. (17) found significant differences between fusion constructs supplemented with BPS using TLIF and XLIF approaches with larger interbody implants (26 mm, anterior-posterior width); and, with standard 18-mm implants, the XLIF construct demonstrated a significant reduction only in axial rotation. In our current study, no significant differences were observed between LLIF and PLIF constructs with BPS in ROM across all motion types; however, a comparison of interbody type (e.g., LLIF or PLIF) with no-LIF demonstrated a differential effect, with greater stabilization with LLIF + BPS vs. no-LIF + BPS than with PLIF + BPS vs. no-LIF + BPS.
Although the facet joint provides axial rotational resistance in the intact lumbar spine, studies have demonstrated that the partial bilateral facetectomy necessary for the PLIF approach does not significantly affect axial stability in a BPS-augmented setting (20). In our study, we observed a significant difference in SZ between LLIF + BPS and PLIF + BPS in axial rotation, suggesting that the larger footprint and lateral placement of the interbody implant used in the LLIF construct may provide better stability than the smaller and anteroposteriorly placed bilateral PLIF device. Therefore, rather than providing facet joint preservation, the larger footprint of the laterally inserted interbody implant in the LLIF approach may contribute to improved stability, in part due to disc space distraction and greater tension on retained ligaments.
Limitations of our study design include the inherent constraints of an in vitro cadaveric model. Such model systems evaluate only the immediate stability of segmental fixation and cannot easily be extrapolated to construct longevity or fusion success. Additionally, the small sample size of the current study may affect the results and limit the generalizability of our findings, particularly given the increased average donor age, heterogeneity of underlying disease processes, and variable bone quality. Further study is needed to clarify our understanding of this model system and to contextualize our findings in clinical practice.
Conclusions
This cadaveric biomechanical study directly compared the immediate postoperative stability between interbody fusion constructs using the LLIF and PLIF approaches in the presence of BPS. For most loading parameters, the LLIF construct demonstrated equivalence to the PLIF construct. Our data indicate that the interbody fusion construct using LLIF with dorsal supplemental fixation is a biomechanically equivalent alternative to conventional dorsal approaches to LIF.
Acknowledgments
The authors thank the staff of Neuroscience Publications at Barrow Neurological Institute for assistance with manuscript preparation. This study was funded by grant support from Medtronic, Inc.
Footnote
Conflicts of Interest: SW Chang receives royalties from Globus Medical, Inc., royalties from Biomet, Inc., consulting fees from LDR Holding Corp., and educational grants from Medtronic, Inc. V Singh is an employee of Medtronic, Inc. NR Crawford is an employee of Globus Medical, Inc. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors warrant that this research conformed to the ethical principles of the Declaration of Helsinki. Neither institutional review board approval nor consent was deemed necessary due to the cadaveric nature of the study.
References
- Cappuccino A, Cornwall GB, Turner AWL, et al. Biomechanical analysis and review of lateral lumbar fusion constructs. Spine (Phila Pa 1976) 2010;35:S361-S7. [Crossref] [PubMed]
- Arnold PM, Anderson KK, McGuire RA Jr. The lateral transpsoas approach to the lumbar and thoracic spine: a review. Surg Neurol Int 2012;3:S198-215. [Crossref] [PubMed]
- Oliveira L, Marchi L, Coutinho E, et al. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976) 2010;35:S331-7. [Crossref] [PubMed]
- Ozgur BM, Aryan HE, Pimenta L, et al. Extreme Lateral Interbody Fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 2006;6:435-43. [Crossref] [PubMed]
- Crawford N, Brantley A, Dickman C. An apparatus for applying pure nonconstraining moments to spine segments in vitro. Spine (Phila Pa 1976) 1995.2097-100. [Crossref] [PubMed]
- Perez-Orribo L, Kalb S, Reyes PM, et al. Biomechanics of lumbar cortical screw-rod fixation versus pedicle screw-rod fixation with and without interbody support. Spine (Phila Pa 1976) 2012;38:635-41. [Crossref] [PubMed]
- Panjabi M. Biomechanical evaluation of spinal fixation devices, part I: a conceptual framework. Spine (Phila Pa 1976) 1988;13:1129-34. [Crossref] [PubMed]
- Panjabi M, Abumi K, Duranceau J. Biomechanical evaluation of spinal fixation devices, part II: stability provided by eight internal fixation devices. Spine (Phila Pa 1976) 1988.1135-40. [Crossref] [PubMed]
- Crawford N, Dickman C. Construction of local vertebral coordinate systems using a digitizing probe: technical note. Spine (Phila Pa 1976) 1997.559-63. [Crossref] [PubMed]
- Crawford N, Yamaguchi G, Dickman C. A new technique for determining 3-D joint angles: the tilt/twist method. Clin Biomech (Bristol, Avon) 1999.153-65. [Crossref] [PubMed]
- Crawford N, Peles J, Dickman C. The spinal lax zone and neutral zone: measurement techniques and parameter comparisons. J Spinal Disord 1998.416-29. [PubMed]
- Steffen T, Tsantrizos A, Aebi M. Effect of implant design and endplate preparation on the compressive strength of interbody fusion constructs. Spine (Phila Pa 1976) 2000;25:1077-84. [Crossref] [PubMed]
- Fogel GR, Turner AWL, Dooley ZA, et al. Biomechanical stability of lateral interbody implants and supplemental fixation in a cadaveric degenerative spondylolisthesis model. Spine (Phila Pa 1976) 2014;39:E1138-E46. [Crossref] [PubMed]
- Harris BM, Hilibrand AS, Savas PE, et al. Transforaminal lumbar interbody fusion: the effect of various instrumentation techniques on the flexibility of the lumbar spine. Spine (Phila Pa 1976) 2004;29:E65-E70. [Crossref] [PubMed]
- Marulanda GA, Nayak A, Murtagh R, et al. A cadaveric radiographic analysis on the effect of extreme lateral interbody fusion cage placement with supplementary internal fixation on indirect spine decompression. J Spinal Disord Tech 2014;27:263-70. [Crossref] [PubMed]
- Sim HB, Murovic JA, Cho BY, et al. Biomechanical comparison of single-level posterior versus transforaminal lumbar interbody fusions with bilateral pedicle screw fixation: segmental stability and the effects on adjacent motion segments. J Neurosurg Spine 2010;12:700-8. [Crossref] [PubMed]
- Pimenta L, Turner AW, Dooley ZA, et al. Biomechanics of lateral interbody spacers: going wider for going stiffer. TheScientificWorldJournal 2012;2012. [Crossref] [PubMed]
- Laws C, Coughlin D, Lotz J, et al. Direct lateral approach to lumbar fusion is a biomechanically equivalent alternative to the anterior approach. Spine (Phila Pa 1976) 2012;37:819-25. [Crossref] [PubMed]
- Fogel GR, Parikh RD, Ryu SI, et al. Biomechanics of lateral lumbar interbody fusion constructs with lateral and posterior plate fixation. J Neurosurg Spine 2014;20:291-7. [Crossref] [PubMed]
- Abumi K, Panjabi MM, Kramer KM, et al. Biomechanical evaluation of lumbar spinal stability after graded facetectomies. Spine (Phila Pa 1976) 1990;15:1142-7. [Crossref] [PubMed]


