
The combined administration of vancomycin IV, standard prophylactic antibiotics, and vancomycin powder in spinal instrumentation surgery: does the routine use affect infection rates and bacterial resistance?
Introduction
Surgical site infections (SSI’s) constitute approximately 20% of all nosocomial infections incurring $3–10 billion in costs (1,2). The 2013 NASS Evidence-Based Clinical Guidelines found that the incidence of SSI in spine surgery ranged from 0.7–10%, with higher rates in patients with medical comorbidities including diabetes mellitus, obesity, and poor hygiene (3,4). Decreased quality of life and function, neurologic injury, failure of instrumentation, pseudarthrosis, progressive instability and deformity, need for additional surgeries, sepsis, and death can occur as a consequence of spine SSI. Spine SSI may lead to profound clinical and economic consequences due to re-hospitalizations, re-operations, long-term antibiotic treatment, untoward side effects of the antibiotics, and continued pain and disability.
The majority of spinal SSI’s are caused by gram-positive organisms, specifically Staphylococcus aureus and Staphylococcus epidermidis, and approximately 34% of all cultured organisms are methicillin-resistant (5). Gram-negative organisms associated with spinal SSI’s are cefazolin-resistant in nearly 60% of cases and are predominantly associated with lumbosacral procedures (5). As such, various broad-spectrum perioperative antibiotic protocols have been proposed to reduce SSI, but despite these modifications in prophylactic regimens, infections with aforementioned organisms persists (6,7). There are concerns that the routine use of broad spectrum antibiotics peri-operatively may produce strains of antibiotic resistant bacteria that may be difficult to treat and eradicate. Particular attention has been dedicated to vancomycin powder recently and multiple meta-analyses demonstrated significant risk and rate reductions in SSIs when used during spine surgeries (8-10). In complex spine surgery with inherently greater risk of infection, the utilization of vancomycin powder yielded a 3.2% infection rate (11).
Currently, the North American Spine Society (NASS) Evidence-Based Clinical Guidelines recommend (Grade C recommendation) the use of IV perioperative antibiotics to prevent SSI in spine implant surgery (3). However, studies do not specify which antibiotic, combination of antibiotics, or duration of antibiotic coverage would be most efficacious in spinal SSI prevention (3,6). In reaction to an increase in methicillin-resistant Staphylococcus aureus (MRSA) infections at this study’s institution, spine surgeries requiring spinal instrumentation have routinely utilized the combination strategy of vancomycin IV in addition to a standard perioperative intravenous antibiotic. The purpose of this study is to report the SSI rate when utilizing this practice and secondarily, to determine if this practice promotes the emergence of antibiotic resistant bacteria.
Methods
This study is a single-center, retrospective case-control study utilizing the electronic medical record (EMR) database. The Institutional Review Board (IRB # 15-001557) provided approval and ethical oversight of this study.
Inclusion criterion for this study was any age subject undergoing spine surgery for any indication that utilized spinal instrumentation, the combination use of vancomycin IV in addition to standard antibiotic IV prophylactically, and at least 90 days of follow-up. Non-instrumented spine surgeries, prophylaxis without combination IV antibiotics, and subjects without 90 days of follow-up were excluded.
The primary outcome of this study was the occurrence of an SSI, as defined by the Center for Disease Control (CDC). The CDC definitions of SSI are summarized as follows (12):
- Superficial SSI are skin or subcutaneous tissue infections within 30 days of the procedure in addition to purulent drainage, cultured organisms, procedural intervention due to pain, swelling, erythema, or heat, or diagnosis of superficial SSI made by the surgeon;
- Deep incisional SSI are infections involving the deep soft tissue (e.g., fascial/muscle layers) in addition to purulent drainage, spontaneous dehiscence or opened wound by surgeon with positive cultures, spontaneous dehiscence or opened wound by surgeon without positive cultures but with fever or pain, or an abscess detected on exam or imaging test.
The secondary outcome of this study was the identification of any antibiotic resistant organisms demonstrated through the culture of SSIs.
All spine surgeries performed at this institution from 2013–2016 were reviewed. A consecutive list of all spinal instrumentation surgeries was identified during this time period. A chart review was then performed to identify the appropriate inclusion and exclusion criteria, demographic data, diagnosis, type of surgery performed, as well as the outcomes of interest. Concurrently, all SSIs were identified and reviewed in 2010 at this institution to serve as the control group since the combined strategy was implemented after 2010.
A Student t-test was used for normally distributed continuous variables. Pearson χ2 analysis was used for comparison of categorical data. Statistical analyses were completed utilize Stata Software (StataCorp., College Station, TX, USA).
Results
Demographic data
One thousand and seventy four subjects met inclusion criteria upon chart review in the combined cohort. With an average age of 52.3 years, 540 subjects were male (50.2%) and 534 were female (49.8%). Nine hundred sixty cases (89.4%) were primary, and 114 cases (10.6%) were revision surgeries. Cervical myelopathy (27.9%), lumbar stenosis (16.2%), lumbar spondylolisthesis (14.0%), and scoliosis (pediatric and adult)/deformity (13.7%) were the leading indications for surgery (Table 1). Posterior lumbar instrumentation accounted for 24.8% of cases, anterior cervical instrumentation 22.6%, posterior cervical instrumentation in 20.9%, and posterior thoracic to lumbar instrumentation comprised of 15.1% of cases in this series (Table 2).
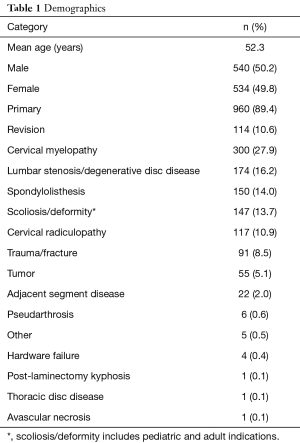
Full table
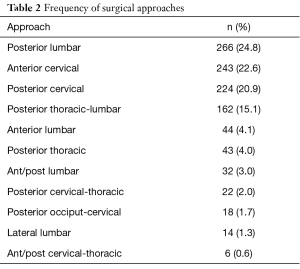
Full table
Prophylactic antibiotic use
Of the 1,074 cases that utilized vancomycin IV with an additional standard prophylactic antibiotic, the additional standard prophylactic antibiotic was cefazolin IV in 524 cases (48.80%), gentamicin IV in 526 cases (49.00%), clindamycin IV in 13 cases (1.21%), cefazolin IV with gentamicin IV in 7 cases (0.65%), cefoxitin IV in 2 cases (0.19%), cefepime in 1 case (0.09%), and piperacillin/tazobactam IV in 1 case (0.09%) (Table 3).
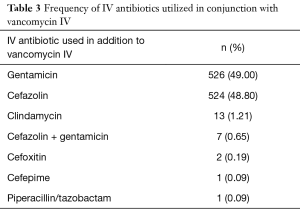
Full table
Vancomycin powder use rates
Vancomycin powder was utilized in the majority (72.3%) of cases (Table 4). Vancomycin powder was utilized in all four instances of infection.
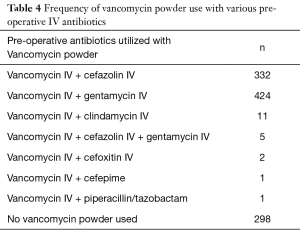
Full table
Infection rates
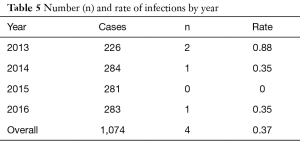
Of the 1,074 cases reviewed, four cases of SSI occurred as defined by the CDC. Two infections in 2013 out of 226 cases (0.88%), one infection in 2014 out of 284 cases (0.35%), zero infections in 2015 out of 281 cases, and one infection in 2016 out of 283 cases (0.35%) occurred during the study period with an overall infection rate of 0.37% (Table 5). In the control group, 11 SSI cases occurred out of 892 cases (1.23%) in 2010, prior to the implementation of the combined strategy. All SSI cases in the control cohort were classified as deep infections and all required irrigation and debridement as secondary procedures as a result of the infections. Five cases utilized cefazolin only and 6 cases utilized vancomycin only. There was no use of vancomycin powder in the cases of the control cohort. All of the SSI cases of the control cohort were primary cases implementing multilevel instrumentation and fusion and the posterior approach was utilized in all of these cases. There were 3 cervical, 3 thoracic, 3 lumbosacral, 1 cervicothoracic, and 1 thoracolumbar case in the control cohort. The difference of SSI rates between the combined antibiotic (0.37%) and control group (1.23%) reached statistical significance (Pearson χ2 test, P<0.05).
Full table
Infection case descriptions and cultured organisms
Case 1: deep incisional SSI
In 2013, a 16-year-old male with a history of neurofibromatosis and severe kyphoscoliosis presented to the emergency room with progressive myelopathy symptoms. The patient underwent a two-stage vertebral column resection and deformity correction. Vancomycin IV and cefazolin IV was utilized pre-operatively, and vancomycin powder was utilized prior to closure. Over the course of the next month, a deep wound dehiscence with exposed implants developed which eventually required flap coverage. Methicillin-sensitive, vancomycin-sensitive Staphylcoccous aureus grew from the wound cultures taken at the time of flap coverage. The patient was treated with an extended course of cefazolin IV and rifampin PO.
Case 2: deep incisional SSI
In 2013, an 84-year-old with a history of hypertension, hyperlipidemia, and GERD with L4–5 spondylolisthesis and stenosis underwent L4–5 laminectomy and fusion. Vancomycin IV and cefazolin IV was utilized pre-operatively, and vancomycin powder was utilized prior to closure. Three weeks following surgery, the patient presented to clinic with deep wound dehiscence and purulent drainage was documented, at which point the patient taken to the operating room for irrigation and debridement. At the time of surgery, only serosanguinous fluid was discovered in the subcutaneous tissue without any involvement deep to the fascia. Three separate cultures from the OR were negative. The patient started a 2-week course of Bactrim and Keflex but 1 week later, she presented to the ED with fevers due to a Pseudomonas urinary tract infection. Given her prior SSI and then readmission for UTI, there was concern for possible hematogenous spread to the spinal instrumentation. The patient was treated with an extended course of intravenous ceftaroline, which was changed to oral doxycycline due to an adverse drug reaction. The patient then made a full recovery without further infection symptoms.
Case 3: deep incisional SSI
In 2014, a 64-year-old male with a history of coronary artery disease and diabetes mellitus who presented with R sided hemiplegia and found to have a mass within the spinal cord extending from C2–C4. The patient underwent a resection, biopsy, and C3–C5 posterior arthrodesis. Vancomycin IV and gentamycin IV was utilized pre-operatively, and vancomycin powder was utilized at closure. Biopsy revealed a Grade II ependymoma. On post-operative day 4, there was evidence of a CSF leak through a focal wound dehiscence and a lumbar drain was placed. On post-operative day 10, the patient was taken to the operating room for an irrigation and debridement and repair of CSF leak. Wound cultures from the I&D grew Klebsiella pneumoniae which was initially treated with vancomycin IV and cefepime IV followed by 2 weeks of Ceftriaxone IV.
Case 4: superficial SSI
In 2016, a 40-year-old male who underwent L4–5 posterior instrumented fusion 5 years prior presented with low back pain and lumbar stenosis at the L5–S1 level. The patient underwent a revision laminectomy and posterior instrumentation and fusion at L4–S1 level. Vancomycin IV and gentamicin IV was utilized pre-operatively, and vancomycin powder was utilized at closure. At the first post-operative visit, a small focal point of dehiscence was seen at the wound with a small amount of purulent discharge. This was cultured and grew methicillin-sensitive Staphylococcus aureus. Pt was treated with a course of Augmentin, and the wound had healed by the next post-operative visit. The patient never exhibited systemic symptoms.
Discussion
The purpose of this study was to report the SSI rate when utilizing the combination of vancomycin IV, standard prophylactic antibiotics and vancomycin powder routinely during spinal instrumentation surgery. This strategy was instituted as a result of numerous MRSA infections at this institution prior to 2013. The secondary purpose of the study was to determine if this routine combination practice promotes the development of antibiotic resistant bacteria. In spinal instrumentation cases that utilized this combined regimen, the surgical site infection rate was 0.37% in the 4-year period studied with no culturing of antibiotic resistant organisms. Of the 4 SSI cases, three cases were deep incisional SSI that required re-operations and long term antibiotic treatment with one superficial SSI that resolved with a short course of oral antibiotics.
Various antibiotics have demonstrated efficacy in spine surgery prophylaxis, but clinical evidence for the pre-operative use of vancomycin IV in combination with standard IV antibiotics is sparse. In a meta-analysis of randomized controlled trials investigating the use of pre-operative prophylactic antibiotics, the use of prophylactic antibiotics demonstrated significant reductions of the rates of SSI (6). Differing regimens utilizing first generation cephalosporins, vancomycin, and gentamicin have evidence to support its use (13-18). Two randomized controlled trials demonstrated greater effectiveness with the use of vancomycin and gentamicin as compared to no prophylaxis in neurosurgical and spine surgeries (19,20). Abdul-Jabbar et al. reported the prevalence of various bacterial organisms in spinal SSI and concluded that cefazolin was a reasonable choice for prophylaxis given that approximately 75% of cultured pathogens were S. aureus or S. epidermidis. However, 34.3% of pathogens were methicillin resistant, and the authors suggested that further research is necessary to investigate the efficacy of adjunctive vancomycin prophylaxis (5). To this point, this present study examined the combined use of vancomycin IV and demonstrated a low rate of SSI using this strategy.
When compared to the control group (1.23% infection rate), the combined group (0.37%) demonstrated significantly lower SSI rates (Pearson χ2, P<0.05). The 2010 cohort was prophylactically treated with a single antibiotic as it was prior to the 2013 implementation of combination antibiotics. The control group infection rate is concordant with previously published infection rates. However, there are several limitations to this study. The effect of vancomycin powder could not be controlled or differentiated with the effect of vancomycin IV. It is possible that the use of vancomycin powder may have a greater local effect in preventing SSI than the systemic administration of vancomycin IV for prophylaxis. A large multicenter observational study of 2,056 patients examined the SSI rates with use of vancomycin powder as compared to no vancomycin use and demonstrated a 2.2% infection rate with vancomycin powder versus 5.1% without (21). Our infection rate of 0.37% was lower than previously published infection rates. The combined utilization of vancomycin IV, standard IV antibiotics, and vancomycin powder may have a synergistic effect, which may further reduce SSI rates as compared to their individual use.
This study is retrospective in design and relatively small in sample size. Given the low rates of SSI, a larger sample size is required to detect greater numbers of infections to substantiate our results and reveal causative relationships. More importantly, larger sample sizes would provide a more accurate representation of the development of vancomycin resistant bacteria using this combined strategy. A large multicenter randomized clinical trial would better elucidate the individual effect of the various interventions, including vancomycin powder as compared to vancomycin IV versus standard prophylactic antibiotics.
To maximize the sample size in this study, all spinal instrumentation surgeries were included despite the heterogeneity of the cases and types of instrumentation used. The anatomic location of the surgery, the surgical approach, and magnitude of surgery may influence the infection risk. Of the 4 SSIs that occurred in our case series, these infection cases involved well established risk factors for post-operative infection, including large deformity surgery, multiple medical comorbidities, and revision surgery.
As part of the inclusion criteria, all subjects had at least 90-day follow-up, however data was not analyzed beyond the 90-day period. Infections may have occurred beyond the first 90 days after surgery. However, the purpose of this study was to evaluate the efficacy of the combined strategy within the early post-operative period. Furthermore, the CDC definition of SSI guided our 90-day analysis. Hematogenous seeding of instrumentation may be a possible cause of late infections, which would not be preventable with surgical prophylaxis.
Despite these limitations, we report low rates of SSI in spine surgeries involving spinal instrumentation within 90 days after surgery. The SSI rate reported in this study is among the lowest reported in the literature. Considering the limitations of this study, we do not posit this study as primary evidence advocating for the routine use of this combined regimen. However, given the considerable clinical and economic consequences of SSI in spinal instrumentation surgery as well as the growing concern for MRSA, this combined strategy of vancomycin IV, standard prophylactic IV antibiotics, and vancomycin powder should serve as a point of consideration for those institutions with substantial rates of SSIs associated with spinal instrumentation and methicillin resistant infections.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Institutional Review Board (IRB # 15-001557) provided approval and ethical oversight of this study.
References
- de Lissovoy G, Fraeman K, Hutchins V, et al. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control 2009;37:387-97. [Crossref] [PubMed]
- Featherall J, Miller JA, Bennett EE, et al. Implementation of an Infection Prevention Bundle to Reduce Surgical Site Infections and Cost Following Spine Surgery. JAMA Surg 2016;151:988-90. [Crossref] [PubMed]
- Shaffer WO, Baisden JL, Fernand R, et al. An evidence-based clinical guideline for antibiotic prophylaxis in spine surgery. Spine J 2013;13:1387-92. [Crossref] [PubMed]
- Olsen MA, Nepple JJ, Riew KD, et al. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am 2008;90:62-9. [Crossref] [PubMed]
- Abdul-Jabbar A, Berven SH, Hu SS, et al. Surgical site infections in spine surgery: identification of microbiologic and surgical characteristics in 239 cases. Spine (Phila Pa 1976) 2013;38:E1425-31. [Crossref] [PubMed]
- Barker FG 2nd. Efficacy of prophylactic antibiotic therapy in spinal surgery: a meta-analysis. Neurosurgery 2002;51:391-400; discussion 400-1. [Crossref] [PubMed]
- Dworsky EM, Hegde V, Loftin AH, et al. Novel in vivo mouse model of implant related spine infection. J Orthop Res 2017;35:193-9. [Crossref] [PubMed]
- Bakhsheshian J, Dahdaleh NS, Lam SK, et al. The use of vancomycin powder in modern spine surgery: a systematic review and meta-analysis of the clinical evidence. World Neurosurg 2015;83:816-23. [Crossref] [PubMed]
- Chiang HY, Herwaldt LA, Blevins AE, et al. Effectiveness of local vancomycin powder to decrease surgical site infections: a meta-analysis. Spine J 2014;14:397-407. [Crossref] [PubMed]
- Khan NR, Thompson CJ, Decuypere M, et al. A meta-analysis of spinal surgical site infection and vancomycin powder. J Neurosurg Spine 2014;21:974-83. [Crossref] [PubMed]
- Van Hal M, Lee J, Laudermilch D, et al. Vancomycin powder regimen for prevention of surgical site infection in complex spine surgeries. Clin Spine Surg 2017;30:E1062-5. [Crossref] [PubMed]
- Horan TC, Gaynes RP, Martone WJ, et al. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 1992;13:606-8. [Crossref] [PubMed]
- Pavel A, Smith RL, Ballard A, et al. Prophylactic antibiotics in elective orthopedic surgery: a prospective study of 1,591 cases. South Med J 1977;70 Suppl 1:50-5. [Crossref] [PubMed]
- Young RF, Lawner PM. Perioperative antibiotic prophylaxis for prevention of postoperative neurosurgical infections. A randomized clinical trial. J Neurosurg 1987;66:701-5. [Crossref] [PubMed]
- Bullock R, van Dellen JR, Ketelbey W, et al. A double-blind placebo-controlled trial of perioperative prophylactic antibiotics for elective neurosurgery. J Neurosurg 1988;69:687-91. [Crossref] [PubMed]
- Djindjian M, Lepresle E, Homs JB. Antibiotic prophylaxis during prolonged clean neurosurgery. Results of a randomized double-blind study using oxacillin. J Neurosurg 1990;73:383-6. [Crossref] [PubMed]
- Rubinstein E, Findler G, Amit P, et al. Perioperative prophylactic cephazolin in spinal surgery. A double-blind placebo-controlled trial. J Bone Joint Surg Br 1994;76:99-102. [Crossref] [PubMed]
- Nishant, Kailash KK, Vijayraghavan PV. Prospective Randomized Study for Antibiotic Prophylaxis in Spine Surgery: Choice of Drug, Dosage, and Timing. Asian Spine J 2013;7:196. [Crossref] [PubMed]
- Shapiro M, Wald U, Simchen E, et al. Randomized clinical trial of intra-operative antimicrobial prophylaxis of infection after neurosurgical procedures. J Hosp Infect 1986;8:283-95. [Crossref] [PubMed]
- Geraghty J, Feely M. Antibiotic prophylaxis in neurosurgery. A randomized controlled trial. J Neurosurg 1984;60:724-6. [Crossref] [PubMed]
- Devin CJ, Chotai S, McGirt MJ, et al. Intrawound vancomycin decreases the risk of surgical site infection after posterior spine surgery-A multicenter analysis. Spine (Phila Pa 1976) 2018;43:65-71. [Crossref] [PubMed]


