
Effect of interbody fusion cage on clinical and radiological outcome of surgery in L4–L5 lumbar degenerative spondylolisthesis
Introduction
Lumbar degenerative spondylolisthesis (LDS) is a degenerative slippage of a lumbar vertebra relative to the adjacent vertebra below that can be clinically symptomatic. Due to intact neural arch, slip percentage is usually less than 30–40 or neurologic symptoms will be severe (1). The disease usually affects 5–7% of the general population and is more common in L4–L5 level of the older women (2,3).
Most of the patients with LDS clinically have no or very few symptoms that do not affect their normal activities of daily living. Those patients become symptomatic mostly give an appropriate response to conservative treatment but surgical intervention is sometime necessary in refractory cases (4). The aim of surgery is to remove pressure on the neural elements and, if necessary, stabilize the spine in the shortest possible time with minimal morbidity. In the surgical treatment of LDS, a variety of techniques including decompression, vertebral reduction, fusion (posterolateral or interbody), and instrumentation (pedicle screw, rod, plate, interspinous device, etc.) have been used alone or together with no universal agreement (5-10).
With the entry of a variety of interbody cages to the market, it was proposed that these added materials could improve the fusion and prevent from loss of reduction. In this study, we aim to compare radiological and clinical outcome of surgery in L4–L5 LDS with or without applying the interbody fusion cage.
Methods
After local institutional review board approval (record No. 940342), we retrospectively studied the patients that had been operated in our department due to L4–L5 LDS from the October 2009 to September 2014. We included those patients with single level L4–L5 LDS who undergone neural decompression, posterolateral fusion (PLF) and instrumentation with or without interbody arthrodesis as a primary operation. We excluded multiple level spondylolisthesis, spondylolisthesis other than degenerative type, spondylolisthesis at other levels, follow-up period less than two years, previous history of lumbar spine surgery, and those patients operated with decompression alone, stand-alone cage or un-instrumented fusion. We divided patients into two groups without and with interbody fusion cage (groups A and B, respectively).
Looking at the number of cases in similar articles and according to the formula test of two means of a quantitative trait in two independent groups, 30 patients in each group was calculated. Therefore, we randomly chose 30 patients for each group among our eligible cases.
Surgical technique
We operated all the patients with a similar technique and by a single surgeon (Farzad Omidi-Kashani) throughout these years. In prone position, while maintaining lumbar lordosis, a midline longitudinal incision was carried out followed by L4 laminectomy, medial partial facetectomy, foraminotomy were performed. Posterolateral gutter was carefully cleaned off the soft tissues and then osseous surfaces decorticated and fused with a mixture of matchstick allograft (10 pieces of 5×5×35 mm3 of freeze-dried cortical cancellous matchstick (Tissue Regeneration Co., Kish, Iran) and autograft obtained from laminectomy. We used polyaxial pedicle screw system (XIA titanium spinal system, Stryker, USA) to reduce and stabilize the slipped vertebra (9). In group B, after completely prepared the intervertebral space, a banana PEEK (polyetheretherketone) cage was also inserted transforaminally to fuse this particular space and stabilize the vertebra more efficiently [transforaminal lumbar interbody fusion (TLIF)] (11).
Clinical and radiologic assessment
We assessed the medical records for evaluating any significant intraoperative events (incidental durotomy, blood loss, operative time, etc.). In coordination with the relevant company (local Stryker representative), the final cost of the implant for each patient was also extracted. Clinical assessment was carried out with visual analog scale (VAS, a 0–10 numerical rating scale) and the Oswestry Disability Index questionnaire (ODI, ver. 2.1), preoperatively and at the last follow-up visit (12-14). Satisfaction rate was assessed at the last visit based on the criteria of the North American Spine Society Low Back Outcome Instrument (15). We took standing radiographies and magnetic resonance imaging scans of the lumbosacral spine, preoperatively. At the last visit, plain upright anteroposterior and lateral radiographs were taken to determine bone bridging in inter-transverse and intervertebral spaces. Complete absence of continuous bone bridge, peripheral radiolucency around the screw or cage, more than 10 degrees motion on dynamic views, or screw breakage were our primary criteria for diagnosis of pseudoarthrosis. We did not use computed tomography (CT) scanning routinely in our patients except in those who were clinically symptomatic. These radiographies were also used to compare slip percentage with preoperative and immediate postoperative radiographs.
Statistical analysis
We used statistical package for social sciences (SPSS), version 16 (SPSS Inc., Chicago, IL, USA) for all statistical analysis. Data were expressed at mean ± SD and number (percent) by descriptive statics. Differences of some variables in two groups were judged by t-test and cross-tab. A two-tailed P value less than 0.05 was considered statistically significant. Some relations between variables such as satisfaction, ODI, VAS and weight of patients were calculated by Spearman’s rho (P<0.05 as significant).
Results
Two groups were homogenous in terms of age, sex, and duration of follow-up. Table 1 demonstrated the demographic data and other related characteristics of our treated patients. This table showed significant that using an interbody fusion cage was associated with a significant increase in intraoperative blood loss, operative time, and instrument cost.
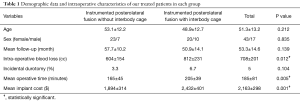
Full table
Clinical and radiological outcome of our treated patients were also depicted and compared in Tables 2 and 3. Mean improvement in VAS (leg or back) and ODI were comparable in two groups and application of cage could not show a superior clinical effect. Loss of reduction and intervertebral union were more favorable in TLIF group but these differences were not statistically significant. Loss of reduction was associated with a raise in dissatisfaction rate of the patients but this correlation was not significant (P=0.72). Spearman’s rank correlation coefficient showed that final satisfaction rate had a negative correlation with ODI, VAS in leg and VAS in back at the last follow-up visit with P=0.008, 0.004, and 0.006, respectively. Patient satisfaction in groups A and B were excellent in 18 and 16, good in 11 and 10, and fair in 1 and 4 patients, respectively. Global satisfaction rate seems higher in group A, although this difference was not significant (P=0.55).
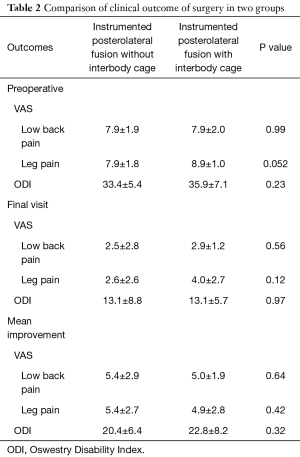
Full table
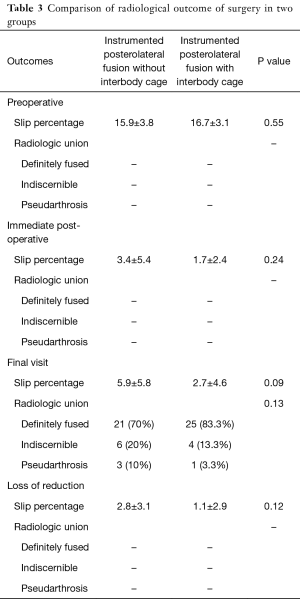
Full table
Discussion
There is an old famous Persian expression that says when an item is new to the market the older one will be heart-rending. This proverb seems completely match with the case of interbody fusion cage in spine surgery. In the early entry of cages into spine marketing, some authors recommended them for all of the lumbar fusions and it was thought to be legit of the previous problems. Although this idea may be true in some cases but it should be greatly investigated and its pros and cons compared. In this study, we assessed efficacy of this intervertebral cages in surgical outcome of L4–L5 LDS and found that application of these cages in especial group of patients, probably not only don’t improve the clinical outcome, but also may raise the complication rate and morbidity.
As the plain radiography has a relative low cost and widespread availability, this modality is commonly used for primary evaluation of bony union, but it is not as accurate as CT scanning. Kim et al. found that a mean time of 3.5 years is needed before the radiologic diagnosis of pseudoarthrosis can be labeled (16). According to Dickson et al. plain radiography alone can detect pseudoarthrosis in 72% of the cases during the first 2 years after surgery (17). These two studies support the idea that plain radiography alone as we used, is not an effective tool for diagnosis of pseudoarthrosis.
Although theoretically it is proposed that interbody fusion due to more extensive contact surface and withstanding compression forces, yields higher rate of bony consolidation compared with posterolateral arthrodesis, this concept is neither universally accepted nor necessarily associated with better clinical outcome. For example, Farrokhi et al. in a randomized prospective study on 80 cases with isthmic spondylolisthesis compared surgical results of PLF with posterior lumbar interbody fusion (PLIF) (18). Fusion rate was significantly better in PLIF group but clinically, improvement in low back pain was more significant in PLF group. The authors proposed that in surgical treatment of spondylolisthesis, better radiologic union does not guarantee better clinical outcome. Similarly, Pooswamy et al. in a retrospective study compared surgical outcomes of TLIF and instrumented PLF in 40 patients with low-grade spondylolisthesis who had been followed-up for 3 years. They found similar clinical and radiologic outcome in both groups except more operating time in TLIF group (19).
In another study, Challier and co-authors in a monocentric open-label randomized controlled trial study on 60 patients with one-level LDS, compared PLF and TLIF during 2 years follow-up (20). Similar to our study, although intragroup improvement in clinical parameters were significant, intergroup comparison did not show difference. Fusion rate was higher in TLIF group but segmental lordosis improvement was comparable. These authors did not find interbody fusion necessary in surgical treatment of these especial patients.
Müslüman at 2011 in a retrospective study evaluated clinical and radiologic efficacy of PLIF and PLF in 50 patients with low-grade spondylolisthesis who were followed-up for 3.3 years (21). They reported good or excellent clinical results in 88% and 79% of the PLIF and PLF patients, respectively. Fusion rate and improvement in lumbar lordosis were both significantly greater in PLIF patients without adding complication rates. These authors recommended PLIF vs. PLF in adults with low-grade spondylolisthesis. In the study we carried out, we could not find such priority in the patients treated with interbody fusion cage. In comparing PLIF and PLF, Liu and co-authors carried out a systematic review with meta-analysis in 2014 (22). They found that moderate-quality evidence suggests superiority of PLIF relative to PLF in term of patient satisfaction and fusion rate, although the complication rate, intraoperative blood loss, and operative time were comparable, statistically.
Adding interbody fusion into the routine surgical procedure of lumbar spondylolisthesis may seem more essential in high-grade spondylolisthesis, spondylolisthesis with associated significant kyphotic or scoliotic deformity, high disc space height, or osteoporosis (23-26). In these cases, the presence of an anterior structural support (cage) could be significantly helpful in maintaining the reduced vertebra in ideal position until the bony union occurs. In support of this issue, it is better to point out the study conducted by Dehoux et al. in 2004 (23). The authors carried out a prospective study on 52 patients with different grade of isthmic spondylolisthesis who had been treated with PLF and PLIF. Clinical and radiologic outcome were comparable in low-grade patients while, surgical outcome decreased in those patients with high-grade spondylolisthesis who had been treated with PLF. These authors recommended PLIF in the cases with high-grade spondylolisthesis who require slip reduction or has a high disc space height.
Although our study was a monocentric study conducted by a single surgical team with a similar technique throughout the study, and our intended patients were homogenous (low-grade L4–L5 LDS), we had some important flaws that deserve mentioning. Firstly, we did not perform post-operative CT scanning routinely to prove bony fusion more efficiently. Therefore, we could not present a more accurate analysis on fusion rate. And second, we could not perform a longer-term follow-up of the patients. Simultaneous with the advent of late post-operative complications (including adjacent segment disease, implant failure, etc.) the results may be different. In conclusion, in the surgical treatment of the patients with L4–L5 LDS that the slip percentage and disc space height are usually low and local segmental kyphosis is usually trivial, interbody fusion cage probably does not significantly improve the radiologic and clinical outcomes and may also be associated with more complication and morbidity.
Acknowledgements
The authors thank Student Research Committee, Faculty of Medicine, Mashhad University of Medical Sciences for financial support. This paper is based on an orthopedic resident’s thesis (Reza Jalilian).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The manuscript was the result of a proposal research with ID number of 940342 with regional ethical approval.
References
- Majid K, Fischgrund JS. Degenerative lumbar spondylolisthesis: trends in management. J Am Acad Orthop Surg 2008;16:208-15. [Crossref] [PubMed]
- Jacobsen S, Sonne-Holm S, Rovsing H, et al. Degenerative lumbar spondylolisthesis: an epidemiological perspective: the Copenhagen Osteoarthritis Study. Spine 2007;32:120-5. [Crossref] [PubMed]
- Enyo Y, Yoshimura N, Yamada H, et al. Radiographic natural course of lumbar degenerative spondylolisthesis and its risk factors related to the progression and onset in a 15-year community-based cohort study: the Miyama study. J Orthop Sci 2015;20:978-84. [Crossref] [PubMed]
- Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med 2007;356:2257-70. [Crossref] [PubMed]
- Eismont FJ, Norton RP, Hirsch BP. Surgical management of lumbar degenerative spondylolisthesis. J Am Acad Orthop Surg 2014;22:203-13. [Crossref] [PubMed]
- Steiger F, Becker HJ, Standaert CJ, et al. Surgery in lumbar degenerative spondylolisthesis: indications, outcomes and complications. A systematic review. Eur Spine J 2014;23:945-73. [Crossref] [PubMed]
- Kleinstueck FS, Fekete TF, Mannion AF, et al. To fuse or not to fuse in lumbar degenerative spondylolisthesis: do baseline symptoms help provide the answer? Eur Spine J 2012;21:268-75. [Crossref] [PubMed]
- Hashimoto T, Shigenobu K, Kanayama M, et al. Clinical results of single-level posterior lumbar interbody fusion using the Brantigan I/F carbon cage filled with a mixture of local morselized bone and bioactive ceramic granules. Spine 2002;27:258-62. [Crossref] [PubMed]
- Omidi-Kashani F, Hasankhani EG, Heidari H. Reduction versus in situ fusion in surgical treatment of lumbar degenerative spondylolisthesis. Intern Res Med Scien 2014;2:92-6.
- Joaquim AF, Milano JB, Ghizoni E, et al. Is There a Role for Decompression Alone for Treating Symptomatic Degenerative Lumbar Spondylolisthesis?: A Systematic Review. Clin Spine Surg 2016;29:191-202. [Crossref] [PubMed]
- Omidi-Kashani F, Hasankhani EG, Noroozi HR. Instrumented transforaminal lumbar interbody fusion in surgical treatment of recurrent disc herniation. Med J Islam Repub Iran 2014;28:124. [PubMed]
- Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health 1990;13:227-36. [Crossref] [PubMed]
- Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976) 2000;25:2940-52. [Crossref] [PubMed]
- Mousavi SJ, Parnianpour M, Mehdian H, et al. The Oswestry Disability Index, the Roland-Morris Disability Questionnaire, and the Quebec Back Pain Disability Scale: translation and validation studies of the Iranian versions. Spine (Phila Pa 1976) 2006;31:E454-9. [Crossref] [PubMed]
- Wood EG III, Hanley EN Jr. Lumbar disc herniation and open limited discectomy: indications, techniques, and results. Oper Tech Orthop 1991;1:23-8. [Crossref]
- Kim YJ, Bridwell KH, Lenke LG, et al. Pseudarthrosis in adult spinal deformity following multisegmental instrumentation and arthrodesis. J Bone Joint Surg Am 2006;88:721-8. [PubMed]
- Dickson DD, Lenke LG, Bridwell KH, et al. Risk factors for and assessment of symptomatic pseudarthrosis after lumbar pedicle subtractionosteotomy in adult spinal deformity. Spine 2014;39:1190-5. [Crossref] [PubMed]
- Farrokhi MR, Rahmanian A, Masoudi MS. Posterolateral versus posterior interbody fusion in isthmic spondylolisthesis. J Neurotrauma 2012;29:1567-73. [Crossref] [PubMed]
- Pooswamy S, Muralidharagopalan NR, Subbaiah S. Transforaminal lumbar interbody fusion versus instrumented posterolateral fusion in Grade I/II spondylolisthesis. Indian J Orthop 2017;51:131-8. [Crossref] [PubMed]
- Challier V, Boissiere L, Obeid I, et al. One-Level Lumbar Degenerative Spondylolisthesis and Posterior Approach: Is Transforaminal Lateral Interbody Fusion Mandatory?: A Randomized Controlled Trial With 2-Year Follow-Up. Spine 2017;42:531-9. [Crossref] [PubMed]
- Müslüman AM, Yılmaz A, Cansever T, et al. Posterior lumbar interbody fusion versus posterolateral fusion with instrumentation in the treatment of low-grade isthmic spondylolisthesis: midterm clinical outcomes. J Neurosurg Spine 2011;14:488-96. [Crossref] [PubMed]
- Liu X, Wang Y, Qiu G, et al. A systematic review with meta-analysis of posterior interbody fusion versus posterolateral fusion in lumbar spondylolisthesis. Eur Spine J 2014;23:43-56. [Crossref] [PubMed]
- Dehoux E, Fourati E, Madi K, et al. Posterolateral versus interbody fusion in isthmic spondylolisthesis: functional results in 52 cases with a minimum follow-up of 6 years. Acta Orthop Belg 2004;70:578-82. [PubMed]
- Goyal N, Wimberley DW, Hyatt A, et al. Radiographic and clinical outcomes after instrumented reduction and transforaminal lumbar interbody fusion of mid and high-grade isthmic spondylolisthesis. J Spinal Disord Tech 2009;22:321-7. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2-18. [PubMed]
- Takahashi T, Hanakita J, Watanabe M, et al. Lumbar alignment and clinical outcome after single level asymmetrical transforaminal lumbar interbody fusion for degenerative spondylolisthesis with local coronal imbalance. Neurol Med Chir (Tokyo) 2014;54:691-7. [Crossref] [PubMed]


