
Platelet-rich plasma injections: an emerging therapy for chronic discogenic low back pain
Introduction
Discogenic low back pain
Low back pain is one of the major causes of physical disability affecting both older and younger people and can have enormous socioeconomic and health impacts. One of the major causes of low back pain is age-associated intervertebral disc degeneration (1,2), which affects the nervous system around the disc. Stimulation of the nociceptors in the annulus fibrosus causes pain, which is termed “discogenic” pain (3). Interestingly, degeneration, endplate injury and inflammation can stimulate pain receptors inside the disc, leaving the external disc intact (4). Intervertebral disc degeneration can be described as an active process involving changes in tissue and the cellular microenvironment that eventually lead to structural breakdown and impairment of intervertebral disc function (5).
Reported pathologic features of painful discs include the formation of zones of vascularized granulation tissue with extensive innervation in annular fissures (6). Due to the avascular nature of intervertebral discs and, hence, their limited ability to regenerate, research on the regeneration of intervertebral discs and the various associated treatment methods has increased. Raj et al. [2008] (7) reported that various biochemical changes occur during disc degeneration, including loss of proteoglycan, loss of collagen fibers, increased fibronectin, increased enzymatic activity, increased fragmentation of collagen, proteoglycan and fibronectin, and changes in nutritional pathways. Histologic examination of painful discs has revealed the formation of a zone of vascularized granulation tissue extending from the nucleus pulposus to the outer part of the annulus fibrosus along the edges of the annular fissures, and growth of nerves deep into the annulus fibrosus and nucleus pulposus (8).
Disc degeneration is accompanied by changes in the matrixes of both the nucleus pulposus and the inner annulus fibrosus that are mediated by an inflammatory process (9). Nociceptive stimuli include pro-inflammatory cytokines produced by disc cells [such as interleukin (IL)-1, IL-4, IL-6, IL-8, IL-12, IL-17], interferon-γ, tumor necrosis factor (TNF)-α, downstream signaling molecules such as nitric oxide (NO), leukotrienes, prostaglandin E and by-products of disc cell metabolism such as lactic acid (9). Disc degeneration can also be caused by aging, apoptosis, vascular ingrowth, failure of nutrient supply to disc cells, abnormal mechanical loads or genetic factors (7,10). Rather than simply providing symptomatic relief, it is important to understand the pathophysiology of degenerated discs to determine the most effective treatment of the underlying cause.
Current treatments for discogenic back pain
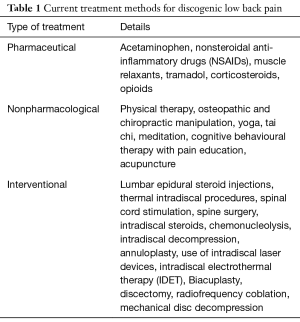
As extensively reviewed by Raj et al. (7) and Simon et al. (11), a number of methods are used for the management of discogenic low back pain (Table 1). Since it is widely believed that degenerated discs are the source of discogenic pain, treatments mostly focus on surgical procedures such as fusion and total disc replacement. The reliability and effectiveness of these surgical procedures are still debated, as they are reported to only offer pain relief (9). Alternatively, non-invasive methods such as benign neglect, physical therapy or symptom control with medication or injection have been employed to treat discogenic pain. Notably, these treatments do not improve the underlying degenerative condition, although they do resolve its symptoms (12). This clearly indicates the need for new therapies and/or interventions that actually treat the underlying causes of discogenic pain. Accordingly, increased attention has been given to emerging techniques such as growth factor therapy, and biomolecular and cellular treatments.
Full table
Previously reported in vitro, in vivo and clinical data clearly demonstrate the effectiveness and feasibility of biomolecular and cellular therapies for treating degenerative disc disease (13-15). Direct injection of growth factors into the annulus fibrosus and nucleus pulposus have resulted in clinically-proven improvement (16). Cellular and biomolecular treatments (which are in the clinical trial stage) combined with tissue engineering and annular repair (which are still in the preclinical stages) have been proposed to have great potential for the treatment of degenerative disc disease (17). Regenerative therapies for degenerated discs should focus on stimulating the production of the extracellular matrix or inhibiting the cytokines that upregulate matrix-degrading enzymes, which in turn may prevent loss of disc space height, increased loading on posterior elements and spinal stenosis (18).
Platelet-rich plasma (PRP)
PRP is defined as autologous blood with platelet concentrations above the physiological baseline. It is obtained by a centrifugation process which separates the liquid and solid components of blood (19,20). In recent years, PRP injections have gained considerable attention as a treatment method for musculoskeletal conditions due to their safety and ability to potentially enhance soft tissue healing. Tissue regeneration in musculoskeletal conditions is achieved by injecting PRP percutaneously. PRP has been effectively used for the treatment of rotator cuff tears, osteoarthritis of the knee, ulnar collateral ligament tears, lateral epicondylitis, hamstring injuries and Achilles tendinopathy (21). However, there is limited data showing its effectiveness for the treatment of intervertebral disc degeneration and low back pain. This article aims to shed light on the use of PRP for treating discogenic low back pain by reviewing the current clinical evidence in human applications.
Repairing effect of PRP
PRP is postulated to promote endogenous healing processes; however, the mechanism remains unclear. It is reported that healing occurs after PRP stimulates the recruitment, proliferation and differentiation of cells involved in regeneration via a number of growth factors and proteins released from the platelets (22). Nonetheless, platelets contain antibacterial proteins and are capable of migrating to injury sites (23). The growth factors released by platelets include vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), transforming growth factor (TGF) β-1, platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF), insulin-like growth factor (IGF)-I, basic fibroblast growth factor (bFGF) and connective tissue growth factor (CTGF), which contribute significantly to tissue proliferation (22,24,25). These growth factors, produced by the concentrated platelets present in PRP, may restore the integrity of the extracellular matrixes of degenerating intervertebral discs (26). A key characteristic of these platelets is that they can release cytokines, chemokines and chemokine receptors and, thus, contribute to the regulation of inflammatory responses and immunological aspects of tissue healing. Platelets also prevent excessive leukocyte recruitment by anti-inflammatory cytokines (27).
PRP and intervertebral disc regeneration
How does PRP inhibit disc degeneration? Disc degeneration is a sequential process possibly starting with a circumferential tear in the annulus fibrosus that progresses to a radial tear, herniation, loss of disc height and resorption (28). In skin wound healing, platelets have the ability to bring disrupted cells closer together. Likewise, platelets pull the edges of degenerated disc tears together, leading to healing of cells. However, this is quite challenging due to the avascular nature of discs, which are not highly vascularized like skin (28).
Existing data on PRP and intervertebral disc degeneration include in vitro studies, in vivo studies, preclinical animal studies and human clinical trials. There is a large amount of evidence for the efficacy of the injection of growth factors for the treatment of intervertebral disc degeneration in animal models (14,29-33). PRP has also proven its efficacy in vivo in the improvement of disc height and disc hydration (17), which has enabled the technology to be used in human clinical trials. The remainder of this review will focus on clinical studies and human applications.
Clinical evidence for PRP treatment of back pain
Clinical evidence for PRP treatment of discogenic low back pain in humans has been reported since 2011 (34). Since then, a limited number of clinical studies have demonstrated the effectiveness of PRP therapy (Table 2). In 2011, Akeda et al. (34) conducted a preliminary clinical trial demonstrating the safety and efficacy of intradiscal injection of autologous PRP as a biological therapy for degenerative disc disease. The study was performed on six patients who suffered chronic low back pain for more than three months. Degenerated discs were confirmed by magnetic resonance imaging (MRI) and standardized provocative discography. At six months follow-up, patients showed a significant decrease in mean pain score and no adverse events were reported post-treatment.

Full table
Bodor et al. [2014] studied 35 patients who were given 47 disc injections of PRP in the lumbar and thoracic spine (28). Two-thirds of the patients showed positive outcomes. The authors also presented a detailed case series of five patients with discogenic back pain treated with PRP injections. The follow-up period ranged from ten days to 10 months, in which patients exhibited substantial improvements in pain that enabled them to return to normal physical activities. Despite two patients having vasovagal episodes, there were no complications or side effects related to this treatment.
In 2016, Levi et al. published data from a prospective clinical trial on 22 patients examining the effect of intradiscal PRP injection on discogenic back pain (35). No complications or serious side effects were reported. Back pain was measured using a visual analogue scale (VAS) and Oswestry Disability Index (ODI). After a 6-month follow-up period, 47% of patients reported at least a 50% improvement in pain and a 30% improvement in their ODI score. The authors speculate that the time frame required for the treatment to take effect, possible adverse effects from the anesthetics and antibiotics used during the procedure, and the PRP preparation method used, account for the lack of a significant positive outcome in this study. In another study by Navani and Hames [2015], six patients were given a single injection of 1.5–3 mL of autologous PRP (36). At a 24-week follow-up, patients reported a 50% decrease in pain according to the verbal pain scale (VPS), with no adverse effects reported.
In 2016, Hussein and Hussein performed a clinical trial on 104 patients with chronic low back pain (37). Unlike the studies mentioned earlier in this section, platelet leucocyte-rich plasma (PLRP) was used instead of PRP, owing to the phagocytic nature of leucocytes. Injections were carried out weekly for 6 weeks. The method was proven to be a safe and effective method for relieving chronic low back pain, with a success rate of 71.2% reported by the authors. No adverse effects or complications were reported other than short-term pain at the injection site.
The first double-blind randomized controlled trial (RCT) of intradiscal PRP therapy was performed by Tuakli-Wosornu et al. in 2016 on 47 participants with chronic lumbar discogenic pain (38). Participants with a history of chronic axial low back pain were recruited and were randomly allocated to treatment or control groups at a 2:1 ratio, respectively. At an 8-week follow-up, outcomes were measured by Functional Rating Index (FRI), Numeric Rating Scale (NRS)-best pain, the Short Form (SF)-36, and modified North American Spine Society (NASS) satisfaction scores. The study found statistically significant improvements in the treatment group, and the effects of PRP were sustained for a period of at least 1 year according to FRI scores. No complications were reported.
In a pilot study performed on ten patients in 2016 by Bhatia and Chopra, PRP injections were shown to improve pain (39). Patients suffering from chronic prolapsed intervertebral discs were given single 5 mL injections of autologous PRP and were followed up after 3 months. Improvement in pain was evaluated using VAS, the Modified Oswestry Disability Questionnaire (MODQ) index and Straight Leg Raising Test (SLRT). All patients had a gradual improvement in symptoms that persisted for at least three months without any complications.
In 2017, Akeda et al. conducted a clinical study investigating the safety and feasibility of autologous PRP releasate injections for discogenic low back pain (40). PRP releasate is a form of bioactive soluble factors isolated from activated PRP that can stimulate tissue repair. The authors implicated that the platelets were isolated by the buffy coat (BC) method and therefore contained lower concentrations of pro-inflammatory cytokines; hence, the sample was considered as “pure PRP”. This prospective, preliminary clinical study was carried out in 14 patients with lumbar discogenic low back pain for a period of 10 months. Seventy-one percent of patients showed a 50% reduction in pain as measured by VAS scores; however, low back pain returned in two patients. In contrast to the VAS scores, physical disability scores [Roland-Morris Disability Questionnaire (RDQ)] were significantly reduced in 79% of patients. Apart from temporary leg numbness in two patients, no other notable adverse events were reported. In summary, this study proved the safety, feasibility and efficacy of PRP in the treatment of lumbar discogenic back pain.
A single case report by Lutz [2017] reported on the effectiveness of intradiscal PRP injection for improving low back pain and function (41). The patient was diagnosed with a degenerated disc and had received an ineffective caudal epidural steroid injection and physical therapy. The patient was given a single PRP injection and showed considerable improvement in pain and motion after 6 weeks. At a 1-year follow-up, there was remarkable improvement in low back pain and the patient was able to return to athletic activities.
The clinical studies discussed so far in this review demonstrate the efficacy of autologous PRP when applied alone in the treatment of chronic back pain. Therefore, a report which shows the effect of PRP injection together with another agent [stromal vascular fraction (SVF)] is particularly interesting. Comella et al. investigated the safety and efficacy of PRP in combination with SVF delivered into the disc nucleus of patients with degenerative disc disease (42). SVF is a mixture of adipose-derived stem cells (ADSCs) and growth factors. The study proved to be safe and successful with significant improvements in flexion, VAS, and pain scores according to the Present Pain Intensity (PPI) scale, SF-12 and Dallas Pain Questionnaires (DPQ). The majority of patients reported remarkable reductions in pain compared to baseline over a period of 6 months post-injection. The only side effects reported were soreness in the abdomen from liposuction (for SVF) and soreness in the back from the PRP injection, both of which resolved within 1 week.
Unpublished clinical data
A search of unpublished and ongoing clinical work identified three clinical trials evaluating PRP injections for the treatment of low back pain. The details of these studies are presented in Table 3.

Full table
Discussion
This review aimed to summarize results from both published and unpublished clinical trials of PRP therapy used in the treatment of discogenic low back pain. The majority of the published clinical studies have applied PRP injections for knee osteoarthritis and epicondylitis, with few reporting its effectiveness for discogenic low back pain. Interestingly, the clinical studies presented here clearly demonstrate the growing interest in PRP injections for treating back pain, with the number of published clinical studies increasing in the past few years. However, it should be noted that there is a lack of RCTs among the reviewed studies (Table 2).
The clinical studies that used PRP injections as a therapy for discogenic low back pain reported good results overall. A major and notable advantage of the therapy is the safety of the autologous PRP itself, which does not cause any major complications. Other than a few temporary side effects (soreness at the injection site, numbness in legs), none of the studies reported any serious adverse events or complications resulting from the injections. Because autologous PRP is obtained from the patient’s own blood, PRP therapy carries low risks of disease infection and allergic reaction (43). In addition, it has been reported that PRP has antimicrobial properties (44,45), which in turn could reduce postsurgical infection risk.
Research on PRP therapy has demonstrated remarkable improvements in pain intensity according to a variety of pain scores. The clinically-beneficial effects have enabled patients to return to normal physical activity (28,41). Notably, the number of injections (single, multiple or at multiple levels), volume of PRP injected (1–5 mL), initial whole blood volume (9–20 mL) and follow-up periods (8 weeks–18 months) varied across the studies. The PRP isolation procedures used in the studies described in this review remained fairly similar. They involved centrifugation of the patients’ whole blood and use of a commercial kit or in-house technique.
Even though the clinical application of PRP injection for degenerated discs is gaining popularity, an important aspect which needs to be considered is the age of the target population. The impact of age on the effectiveness of growth factor injections has been previously discussed (14). Likewise, a low number of functional cells in the intervertebral discs of older patients may hinder the efficacy of PRP injections. The PRP therapy will be more efficient if applied before disc degeneration reaches an advanced stage. Another possible approach will be the use of PRP in combination with cellular therapy, such as the use of nucleus pulposus cells.
The cost-effectiveness of PRP therapy remains controversial. In 2013, Hsu et al. reported that it is more expensive than steroid injections when used in the short-term, but potentially less expensive when used for long-term treatment (20). On the other hand, PRP therapy is widely described as cost-effective as it is autologous in nature, simple to prepare and readily available (33,46,47).
Future directions in PRP therapy include conducting more randomized, controlled and unbiased clinical trials to provide higher quality evidence (48). To the best of our knowledge, only a single randomized controlled clinical trial has been conducted on the effectiveness of PRP injections on discogenic low back pain (38). Further research is necessary to investigate the long-term effects of PRP injections, including possible adverse effects, over longer follow-up periods. A possible future clinical direction would be to compare single and multiple injection regimes within the same study. Other aspects such as the method of preparation of PRP including starting whole blood volume, platelet concentration, PRP composition and amount of PRP injected can be further investigated. Additional research on the above aspects will be advantageous to clinicians in providing better guidance and indications for determining individual patient-based treatment plans and, thus, better clinical outcomes.
Conclusions
In this review, we described clinical evidence from the literature and presented an update on the use of PRP therapy for the treatment of discogenic low back pain. It is evident from our review that PRP is a safe, effective and feasible treatment modality and is evolving as a powerful therapy for the treatment of discogenic back pain. Considering the remarkable progress made already, and the other potential aspects which remain for further investigation, PRP therapy undoubtedly offers new and exciting prospects for the treatment of degenerative disc disease and other musculoskeletal disorders.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vo NV, Hartman RA, Patil PR, et al. Molecular Mechanisms of Biological Aging in Intervertebral Discs. J Orthop Res 2016;34:1289-306. [Crossref] [PubMed]
- Taher F, Essig D, Lebl DR, et al. Lumbar degenerative disc disease: current and future concepts of diagnosis and management. Adv Orthop 2012;2012:970752. [PubMed]
- Choi YS. Pathophysiology of degenerative disc disease. Asian Spine J 2009;3:39-44. [Crossref] [PubMed]
- Zhang YG, Guo TM, Guo X, et al. Clinical diagnosis for discogenic low back pain. Int J Biol Sci 2009;5:647-58. [Crossref] [PubMed]
- Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 2009;48:5-10. [Crossref] [PubMed]
- Peng BG. Pathophysiology, diagnosis and treatment of discogenic low back pain. World J Orthop 2013;4:42-52. [Crossref] [PubMed]
- Raj PP. Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain Pract 2008;8:18-44. [Crossref] [PubMed]
- Peng B, Wu W, Hou S, et al. The pathogenesis of discogenic low back pain. J Bone Joint Surg Br 2005;87:62-7. [Crossref] [PubMed]
- Ito K, Creemers L. Mechanisms of Intervertebral Disk Degeneration/Injury and Pain: A Review. Global Spine J 2013;3:145-52. [Crossref] [PubMed]
- Martin MD, Boxell CM, Malone DG. Pathophysiology of lumbar disc degeneration: a review of the literature. Neurosurg Focus 2002;13:E1. [Crossref] [PubMed]
- Simon J, McAuliffe M, Shamim F, et al. Discogenic low back pain. Phys Med Rehabil Clin N Am 2014;25:305-17. [Crossref] [PubMed]
- Hsieh AH, Yoon ST. Update on the pathophysiology of degenerative disc disease and new developments in treatment strategies. Open Access J Sports Med 2010;1:191-9. [Crossref] [PubMed]
- Bae WC, Masuda K. Emerging technologies for molecular therapy for intervertebral disk degeneration. Orthop Clin North Am 2011;42:585-601. [Crossref] [PubMed]
- Masuda K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur Spine J 2008;17 Suppl 4:441-51. [Crossref] [PubMed]
- Wang SZ, Chang Q, Lu J, et al. Growth factors and platelet-rich plasma: promising biological strategies for early intervertebral disc degeneration. Int Orthop 2015;39:927-34. [Crossref] [PubMed]
- Masuda K, An HS. Prevention of disc degeneration with growth factors. Eur Spine J 2006;15 Suppl 3:S422-32. [Crossref] [PubMed]
- Moriguchi Y, Alimi M, Khair T, et al. Biological Treatment Approaches for Degenerative Disk Disease: A Literature Review of In Vivo Animal and Clinical Data. Global Spine J 2016;6:497-518. [Crossref] [PubMed]
- Mascarinas A, Harrison J, Boachie-Adjei K, et al. Regenerative Treatments for Spinal Conditions. Phys Med Rehabil Clin N Am 2016;27:1003-17. [Crossref] [PubMed]
- Hall MP, Band PA, Meislin RJ, et al. Platelet-rich plasma: Current concepts and application in sports medicine. J Am Acad Orthop Surg 2009;17:602-8. [Crossref] [PubMed]
- Hsu WK, Mishra A, Rodeo SR, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg 2013;21:739-48. [PubMed]
- Mlynarek RA, Kuhn AW, Bedi A. Platelet-Rich Plasma (PRP) in Orthopedic Sports Medicine. Am J Orthop (Belle Mead NJ) 2016;45:290-326. [PubMed]
- DeChellis DM, Cortazzo MH. Regenerative medicine in the field of pain medicine: Prolotherapy, platelet-rich plasma therapy, and stem cell therapy-Theory and evidence. Tech Reg Anesth Pain Manag 2011;15:74-80. [Crossref]
- Zhu Y, Yuan M, Meng HY, et al. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthr Cartil 2013;21:1627-37. [Crossref] [PubMed]
- Sys J, Weyler J, Van Der Zijden T, et al. Platelet-rich plasma in mono-segmental posterior lumbar interbody fusion. Eur Spine J 2011;20:1650-7. [Crossref] [PubMed]
- Anitua E, Andía I, Sanchez M, et al. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J Orthop Res 2005;23:281-6. [Crossref] [PubMed]
- Wang SZ, Rui YF, Tan Q, et al. Enhancing intervertebral disc repair and regeneration through biology: platelet-rich plasma as an alternative strategy. Arthritis Res Ther 2013;15:220. [Crossref] [PubMed]
- Galliera E, Corsi MM, Banfi G. Platelet rich plasma therapy: inflammatory molecules involved in tissue healing. J Biol Regul Homeost Agents 2012;26:35S-42S. [PubMed]
- Bodor M, Toy A, Aufiero D. Disc Regeneration with Platelets and Growth Factors. In: Duarte Lana JFS, Andrade Santana MH, Belangero WD, et al. editors. Platelet-rich plasma: regenerative medicine: sports medicine, orthopaedic, and recovery of musculoskeletal injuries. Berlin, Heidelberg: Springer, 2014:265-79.
- Marfia G, Campanella R, Navone SE, et al. Potential use of human adipose mesenchymal stromal cells for intervertebral disc regeneration: a preliminary study on biglycan-deficient murine model of chronic disc degeneration. Arthritis Res Ther 2014;16:457. [Crossref] [PubMed]
- Kamoda H, Ohtori S, Ishikawa T, et al. The Effect of Platelet-Rich Plasma on Posterolateral Lumbar Fusion in a Rat Model. J Bone Joint Surg Am 2013;95:1109-16. [Crossref] [PubMed]
- Gullung GB, Woodall JW, Tucci MA, et al. Platelet-rich plasma effects on degenerative disc disease: analysis of histology and imaging in an animal model. Evid Based Spine Care J 2011;2:13-8. [Crossref] [PubMed]
- Gui K, Ren W, Yu Y, et al. Inhibitory Effects of Platelet-Rich Plasma on Intervertebral Disc Degeneration: A Preclinical Study in a Rabbit Model. Med Sci Monit 2015;21:1368-75. [Crossref] [PubMed]
- Formica M, Cavagnaro L, Formica C, et al. What is the preclinical evidence on platelet rich plasma and intervertebral disc degeneration? Eur Spine J 2015;24:2377-86. [Crossref] [PubMed]
- Akeda K, Imanishi T, Ohishi K, et al. Intradiscal injection of autologous serum isolated from platelet‐rich‐plasma for the treatment of discogenic low back pain: preliminary prospective clinical trial: GP141. Spine: Affiliated Society Meeting Abstracts 2011.
- Levi D, Horn S, Tyszko S, et al. Intradiscal Platelet-Rich Plasma Injection for Chronic Discogenic Low Back Pain: Preliminary Results from a Prospective Trial. Pain Med 2016;17:1010-22. [PubMed]
- Navani A, Hames A. Platelet-rich plasma injections for lumbar discogenic pain: A preliminary assessment of structural and functional changes. Tech Reg Anesth Pain Manag 2015;19:38-44. [Crossref]
- Hussein M, Hussein T. Effect of autologous platelet leukocyte rich plasma injections on atrophied lumbar multifidus muscle in low back pain patients with monosegmental degenerative disc disease. SICOT J 2016;2:12. [Crossref] [PubMed]
- Tuakli-Wosornu YA, Terry A, Boachie-Adjei K, et al. Lumbar Intradiskal Platelet-Rich Plasma (PRP) Injections: A Prospective, Double-Blind, Randomized Controlled Study. PM R 2016;8:1-10. [Crossref] [PubMed]
- Bhatia R, Chopra G. Efficacy of Platelet Rich Plasma via Lumbar Epidural Route in Chronic Prolapsed Intervertebral Disc Patients-A Pilot Study. J Clin Diagn Res 2016;10:UC05-UC07. [PubMed]
- Akeda K, Ohishi K, Masuda K, et al. Intradiscal Injection of Autologous Platelet-Rich Plasma Releasate to Treat Discogenic Low Back Pain: A Preliminary Clinical Trial. Asian Spine J 2017;11:380-9. [Crossref] [PubMed]
- Lutz GE. Increased Nuclear T2 Signal Intensity and Improved Function and Pain in a Patient One Year After an Intradiscal Platelet-Rich Plasma Injection. Pain Med 2017;18:1197-9. [Crossref] [PubMed]
- Comella K, Silbert R, Parlo M. Effects of the intradiscal implantation of stromal vascular fraction plus platelet rich plasma in patients with degenerative disc disease. J Transl Med 2017;15:12. [Crossref] [PubMed]
- Nagae M, Ikeda T, Mikami Y, et al. Intervertebral disc regeneration using platelet-rich plasma and biodegradable gelatin hydrogel microspheres. Tissue Eng 2007;13:147-58. [Crossref] [PubMed]
- Bielecki TM, Gazdzik TS, Arendt J, et al. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: an in vitro study. J Bone Joint Surg Br 2007;89:417-20. [Crossref] [PubMed]
- Fabbro MD, Bortolin M, Taschieri S, et al. Antimicrobial properties of platelet-rich preparations. A systematic review of the current pre-clinical evidence. Platelets 2016;27:276-85. [Crossref] [PubMed]
- Monfett M, Harrison J, Boachie-Adjei K, et al. Intradiscal platelet-rich plasma (PRP) injections for discogenic low back pain: an update. Int Orthop 2016;40:1321-8. [Crossref] [PubMed]
- Barile A, La Marra A, Arrigoni F, et al. Anaesthetics, steroids and platelet-rich plasma (PRP) in ultrasound-guided musculoskeletal procedures. Br J Radiol 2016;89:20150355. [Crossref] [PubMed]
- Phan K, Mobbs RJ. Systematic reviews and meta-analyses in spine surgery, neurosurgery and orthopedics: guidelines for the surgeon scientist. J Spine Surg 2015;1:19-27. [PubMed]


