
Preoperative steroids do not improve outcomes for intramedullary spinal tumors: a NSQIP analysis of 30-day reoperation and readmission rates
Introduction
Intramedullary tumors account for 8–10% of all spinal cord tumors. Although rare, they are a significant cause of neurological morbidity and mortality, affecting patients of all ages (1). Intramedullary spinal cord tumors (IMSCTs) can be found throughout the entire spinal cord, and are most common in the cervical (33%), followed by the thoracic (26%) and lumbar (24%) regions (2). IMSCTs most commonly present with back or neck pain (3).
Ependymomas and astrocytomas are glial-derived tumors that comprise 80–90% of IMSCTs. Ependymomas are more common in adults whereas astrocytomas are more common in children. Rare lesions include hemangioblastomas, gangliogliomas, germinomas, CNS lymphomas, oligodendrogliomas, cavernomas, fibrosarcomas, peripheral neuroectodermal tumors, and intramedullary metastases (2-4). While most IMSCTs are benign, a subset of certain tumor types, including astrocytomas, can be malignant (2).
When there is an easily identified plane of dissection, gross-total resection (GTR) is the treatment of choice for IMSCTs (5,6). Radiotherapy and chemotherapy can be used for high-grade or infiltrative tumors with high recurrence risk, or when total resection is impossible. For patients undergoing surgery for IMSCT resection, administration of steroids in the weeks leading up to surgery is common to reduce spinal cord edema and lower risk of injury. However, despite initial studies supporting the use of methylprednisolone for spinal cord injuries (7-11), recent studies have recommended against it (12). With their benefit unclear, the use of preoperative steroids remains at the discretion of the surgeon due to a lack of standardized treatment protocols (13,14).
The American College of Surgeons-National Surgical Quality Improvement Program (ACS-NSQIP) is a large, multi-institutional prospectively collected registry of data of many surgery types in a standardized, risk-adjusted database. The ACS-NSQIP is designed to provide valid data for patient outcome improvement, and has been, has shown to decrease morbidity and mortality in participating hospitals (15). In this study, the effect of preoperative corticosteroid administration on 30-day outcomes following surgery for IMSCTs was investigated.
Methods
A retrospective review of the prospectively collected American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database was performed. Data for patients undergoing surgery for intramedullary tumors from 2005 to 2015 was collected from current procedural terminology (CPT) codes 63285 (Laminectomy, intradural, intramedullary, cervical), 63286 (Laminectomy, intradural, intramedullary, thoracic), and 63287 (Laminectomy, intradural, intramedullary, thoracolumbar). The study included the following ICD-9 diagnosis codes 192.2, 192.3,192.8, 192.9, 198.3, 198.4, 225.3, 225.4, 237.5, and 237.6. For cases after the conversion to ICD-10, codes C72.0, C79.49, D32.1, D33.4, and D43.4 were used. Ethical approval was obtained through the institutional review board of the University of Illinois at Chicago. As the ACS-NSQIP database is de-identified and poses no risk to participants, a waiver for consent was granted.
Risk factors and outcome measures
Demographics and medical comorbidities were reviewed, including sex, clinical risk factors, and preoperative functional status. Body mass index (BMI) was calculated using the National Institute of Health conversion formula using height and weight data, and obesity was defined as BMI ≥30 kg/m2. The American Society of Anesthesiology (ASA) physical status classification was binned into groups 1–2 and 3 and above to classify patient fitness prior to surgery. Tumor location was stratified by involvement of the cervical, thoracic, or thoracolumbar regions. For all studied patients, thirty-day clinical outcome data was evaluated, including medical and surgical complications, length of hospital stay, discharge destination, reoperation and readmission rate, and death.
Statistical analysis
Group differences were studied for different variables by unpaired two-tailed Student’s t, Fisher’s Exact, or chi-square tests. Significant values were identified with a P value of less than 0.05. Reviewed comorbidities were only analyzed if present in at least five patients, and data points must have been available in at least 50% of patients to be considered for analysis. As complete data was not available for all cases, percentile values were calculated from the proportion of patients where information was available. Statistics were calculated using SPSS (IBM Corporation, Armonk, NY).
Results
Demographics
A total of 259 patients were reviewed. The mean age was 52.9 years, and 121 patients (47%) were female. One hundred eighty-one patients had benign intramedullary tumors and 78 malignant. The majority of the patients had IMSCTs at thoracic (n=100) or cervical (n=99) levels followed by thoracolumbar (n=39). The most common preoperative risk factors were diabetes, present in 38 (15%) patients, and smoking, present in 47 (18%). 109 (42%) patients were obese, and half of patients were ASA Class I or II (50.1%). 31 (12%) patients were pretreated with steroids in the weeks leading up to surgery.
Clinical outcomes
Risk factors for readmission are described in Table 1. Steroid use was associated with neither the risk of readmission nor reoperation. While no patients receiving steroids were readmitted, 2 required reoperations. No association was found between rates of complications and risk of readmission, however, postoperative complications were significantly more common in patients who ultimately required reoperations (62%, P<0.05, OR: 5.6, 95% CI: 2.21–14.37). Readmitted patients were further significantly more likely to be admitted from places other than home such as nursing homes or rehabilitation facilities (21%, P=0.04). The level of the spine operated upon was also different between the readmitted and not readmitted groups, with surgeries of the thoracic spine significantly associated with risk of readmission (P=0.017). A separate analysis of benign and malignant tumors was performed, which revealed no significant difference between benign and malignant tumors for risk of readmission, reoperation, return to the operating room, or postoperative complications.
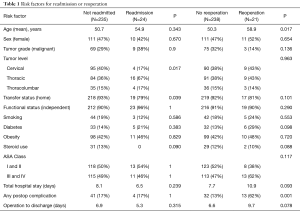
Full table
Corticosteroid use
Risk factors predisposing to pretreatment with steroids and outcomes from steroid use are illustrated in Tables 2,3. Patients in the steroid group were older, on average, than patients not receiving steroids (P=0.02). No significant differences were noted, however, between the steroid and non-steroid groups for tumor malignancy, location, functional status, smoking, diabetic status, rate of obesity, or preoperative ASA Class (Table 2).
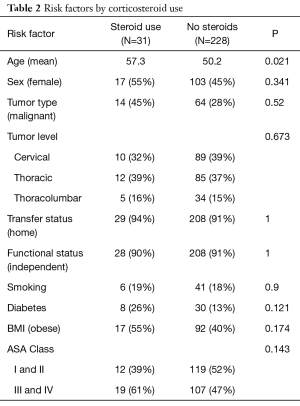
Full table
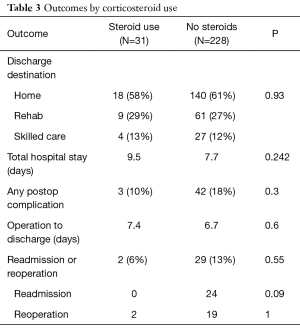
Full table
Outcomes by steroid use are described in Table 3. Steroid use was not predictive of discharge destination (P=0.93). Although the steroid group had a slightly longer mean hospital stay and time between operation and discharge than patients who were not pretreated with steroids, neither of these achieved significance. Complications were low and comparable between both the steroid (10%) and non-steroid groups (18%), suggesting that steroid use does not decrease the complication rate. Rates of readmission and reoperations were higher in the group who did not receive steroids (13%) versus those who did (6%), but this did not achieve significance (P=0.55). Studied individually, readmission rates in the no steroid group approached, but did not achieve, significance (P=0.09). No significant differences were noted between the rates of reoperation between the steroid and no-steroid groups either (P=1).
Readmissions and reoperations
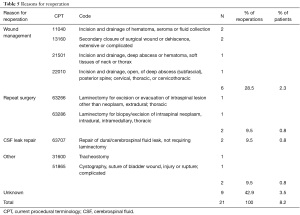
Reasons for readmission are detailed in Table 4. Of 24 readmitted patients, the most common reason was infection, seen in 6, or 2.3% of all patients in the cohort. Non-infectious wound complications were the next most common, seen in 3, followed by vascular occlusions and readmissions related to residual tumor with 2 instances of each. Seven patients, or 2.7% of the cohort, were readmitted but the reason was not mentioned. Cases of reoperation are described in Table 5. The most common reason for reoperation was management of a wound that was infected, dehisced, or had a subcutaneous hematoma or seroma, seen in 6 patients, or 2.3% of the cohort. Two instances of CSF repair, one repeat tumor surgery, and one suspected epidural hematoma evacuation was also noted. In 9 cases, or 3.5% of the cohort, a reoperation was recorded but the reason not specified.
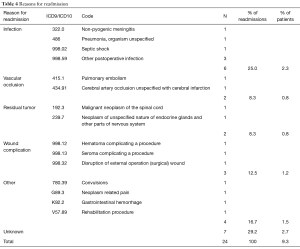
Full table
Full table
Discussion
IMSCTs account for 8–10% of all spinal cord tumors and, although rare, affect patients of all ages and continue to be a source of significant neurological morbidity and mortality (1). When possible, GTR is optimal, but risks iatrogenic injury to the spinal cord (13), in addition to common surgical risks such as cerebrospinal fluid leak, bleeding, infection, and the risks associated with general anesthesia (16). It has been previously suggested that the use of systemic preoperative steroids in patients undergoing IMSCT resection maybe beneficial by decreasing traumatic nerve and spinal cord inflammation (17).
Steroid use in the IMSCT patient
Patients receiving corticosteroids in the weeks leading up to surgery had no significant difference in readmission and reoperation rates in the NSQIP cohort, suggesting steroid administration does not provide a protective effect. Corticosteroids work as an anti-inflammatory agent by suppressing genes involved in the inflammatory response and by activating several anti-inflammatory genes (18). Administering steroids before surgery has therefore been suggested to be helpful by limiting the inflammatory cascade, decreasing swelling and edema (19). Evidence further suggests that administration of steroids can reduce the amount of pain associated with spine surgery (20), facilitating faster recovery and decreasing the risk of readmission or reoperation. The data from this study suggests, however, that in the 30-day period, preoperative steroids are neither beneficial nor harmful for patients undergoing surgery for IMSCTs.
With pretreatment in the weeks prior to surgery very common, why may steroids not provide a benefit in practice? One reason may be infection risk. Chronic steroid use, particularly in patients with additional risk factors, including the elderly, diabetic, and obese, is associated with increased risk of complications. In these populations, additional precautions, such as securing a sterile environment and additional wound irrigation may provide a benefit (18,21-23). Steroids have further been associated with higher rates of medical comorbidities (24), which may contribute to worse 30-day surgical outcomes. Finally, a significant reason why steroids are usually reported as having an effect in the literature may be publication bias: studies that demonstrate a definitive benefit (or harm) of steroids are more likely to be published, while those that show no effect are not. Ultimately, however, the best way to demonstrate the benefit of steroids is through a randomized trial; with no definitive advantage of steroids established, there is sufficient clinical equipoise for trials with both steroid and placebo arms.
Limitations
Studies using national databases, by their nature, carry several limitations. The NSQIP collects data relevant to most surgical patients, including rates of reoperation, readmission, and death, but does not consider outcomes of interest for spine patients, such as neurologic deficits after surgery or improvement of symptoms. This NSQIP cohort is also relatively small given the uncommon nature of IMSCT, but this may be due to strict inclusion and exclusion criteria, and consideration only of patients with complete preoperative and postoperative data.
The definition of the steroid variable also provides a possible limitation. To classify as being on steroids, patients must have received regular doses of corticosteroids for at least 30 days before surgery. While steroids are given to some patients for chronic conditions, like COPD, it is likely that most patients in the NSQIP cohort received steroids as an adjunct in anticipation of surgery. It is conceivable that these patients may have been started on high dose steroids upon IMSCT diagnosis, but, because of the wait times associated with elective surgery, they were on steroid therapy long enough to satisfy NSQIP criteria. A similar rate of medical comorbidities between patients in the steroid and non-steroid groups supports that steroids were stared deliberately in anticipation of surgery, as opposed to chronic use for medical conditions. The patients studied in this paper are, also, by definition, all surgical; a surgical subpopulation means that tumors are more likely to be aggressive, which explains the relatively high rate of malignancy compared to previous reports on intramedullary tumors (2).
Further limitations to this paper include the relatively small percentage of patients on steroids in the sample and a lack of information about the type and dose of steroids used, limiting the ability to generalize the effect of steroid pretreatment between patients. The study is further limited by a lack of information about tumor size, grade, and tissue pathology, which is not described in the patient database.
Conclusions
This study suggests that preoperative systemic corticosteroid use has no significant effect on the 30-day risk of readmission and reoperation for IMSCTs. Preoperative steroid administration is common among patients undergoing surgery for intramedullary tumors; however, due to the lack of clear guidelines, steroid administration to date has been dependent on the preference of the operating surgeon. Although this study by itself is insufficient to establish a clear guideline, future investigation through randomized controlled trials may help further identify the utility of preoperative steroid use for these tumors.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethical approval was obtained through the institutional review board of the University of Illinois at Chicago. As the ACS-NSQIP database is de-identified and poses no risk to participants, a waiver for consent was granted.
References
- Chamberlain MC, Tredway TL. Adult primary intradural spinal cord tumors: a review. Curr Neurol Neurosci Rep 2011;11:320-8. [Crossref] [PubMed]
- Tobin MK, Geraghty JR, Engelhard HH, et al. Intramedullary spinal cord tumors: a review of current and future treatment strategies. Neurosurg Focus 2015;39:E14. [Crossref] [PubMed]
- Samartzis D, Gillis CC, Shih P, et al. Intramedullary Spinal Cord Tumors: Part I-Epidemiology, Pathophysiology, and Diagnosis. Global Spine J 2015;5:425-35. [Crossref] [PubMed]
- Juthani RG, Bilsky MH, Vogelbaum MA. Current Management and Treatment Modalities for Intramedullary Spinal Cord Tumors. Curr Treat Options Oncol 2015;16:39. [Crossref] [PubMed]
- McGirt MJ, Chaichana KL, Atiba A, et al. Neurological outcome after resection of intramedullary spinal cord tumors in children. Childs Nerv Syst 2008;24:93-7. [Crossref] [PubMed]
- Klekamp J. Spinal ependymomas. Part 1: Intramedullary ependymomas. Neurosurg Focus. 2015;39:E6. [Crossref] [PubMed]
- Bracken MB. Methylprednisolone and acute spinal cord injury: an update of the randomized evidence. Spine (Phila Pa 1976) 2001;26:S47-54. [Crossref] [PubMed]
- Fehlings MG. Spine Focus Panel. Summary statement: the use of methylprednisolone in acute spinal cord injury. Spine (Phila Pa 1976) 2001;26:S55. [Crossref] [PubMed]
- Chappell ET. Pharmacological therapy after acute cervical spinal cord injury. Neurosurgery 2002;51:855-6; author reply 856. [Crossref] [PubMed]
- Hurlbert RJ. The role of steroids in acute spinal cord injury: an evidence-based analysis. Spine (Phila Pa 1976) 2001;26:S39-46. [Crossref] [PubMed]
- Bracken MB. Steroids for acute spinal cord injury. Cochrane Database Syst Rev 2012;1:CD001046. [PubMed]
- Hurlbert RJ, Hadley MN, Walters BC, et al. Pharmacological therapy for acute spinal cord injury. Neurosurgery 2013;72 Suppl 2:93-105. [Crossref] [PubMed]
- Verla T, Fridley JS, Khan AB, et al. Neuromonitoring for Intramedullary Spinal Cord Tumor Surgery. World Neurosurg 2016;95:108-16. [Crossref] [PubMed]
- Mehta AI, Mohrhaus CA, Husain AM, et al. Dorsal column mapping for intramedullary spinal cord tumor resection decreases dorsal column dysfunction. J Spinal Disord Tech 2012;25:205-9. [Crossref] [PubMed]
- Montroy J, Breau RH, Cnossen S, et al. Change in Adverse Events After Enrollment in the National Surgical Quality Improvement Program: A Systematic Review and Meta-Analysis. PLoS One 2016;11:e0146254. [Crossref] [PubMed]
- Torpy JM, Lynm C, Golub RM. JAMA patient page. General anesthesia. JAMA 2011;305:1050. [Crossref] [PubMed]
- Glasser RS, Knego RS, Delashaw JB, et al. The perioperative use of corticosteroids and bupivacaine in the management of lumbar disc disease. J Neurosurg 1993;78:383-7. [Crossref] [PubMed]
- Barnes PJ. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br J Pharmacol 2006;148:245-54. [Crossref] [PubMed]
- Livingston EH, Lynm C. JAMA patient page. Steroid injections to treat back pain. JAMA 2012;308:2047. [Crossref] [PubMed]
- Ranguis SC, Li D, Webster AC. Perioperative epidural steroids for lumbar spine surgery in degenerative spinal disease. A review. J Neurosurg Spine 2010;13:745-57. [Crossref] [PubMed]
- McPhee IB, Williams RP, Swanson CE. Factors influencing wound healing after surgery for metastatic disease of the spine. Spine (Phila Pa 1976) 1998;23:726-32; discussion 732-3. [Crossref] [PubMed]
- Lowell TD, Errico TJ, Eskenazi MS. Use of epidural steroids after discectomy may predispose to infection. Spine (Phila Pa 1976) 2000;25:516-9. [Crossref] [PubMed]
- Fang A, Hu SS, Endres N, et al. Risk factors for infection after spinal surgery. Spine (Phila Pa 1976) 2005;30:1460-5. [Crossref] [PubMed]
- Karhade AV, Vasudeva VS, Dasenbrock HH, et al. Thirty-day readmission and reoperation after surgery for spinal tumors: a National Surgical Quality Improvement Program analysis. Neurosurg Focus 2016;41:E5. [Crossref] [PubMed]


