Clinical experience and two-year follow-up with a one-piece viscoelastic cervical total disc replacement
Introduction
Cervical total disc replacement (TDR) began in Europe and Australia in the 1990s. There has been a plethora of TDR designs from the very simple to the very complex in an attempt to simulate normal intervertebral disc function by preserving motion. The success of hip and knee replacement for disorders previously treated with fusion of the joint is at least partially responsible for not only the surge in interest for TDR but many of the early designs. However, hips and knees differ vastly from the intervertebral disc in their energy absorption and kinematics.
The earliest and many of the current cervical TDR designs are uni-articular or bi-articular. Some are metal-on-metal ball-in-trough designs such as the Prestige ST and Prestige LP (Medtronic) and Kineflex-C (Spinal Motion). Others are metal-on-plastic designs with one or two ball-in-socket articulations such as ProDisc C (DePuySynthes), PCM (Nuvasive), Discover (DePuy), Mobi-C (Zimmer Biomet), Secure-C (Globus Medical), ActivC (B Braun) and Synergy (Synergy). The DiscoCerv device (Alphatec) is comprised of titanium plates with a ceramic-on-ceramic articulation. Articulated TDRs have demonstrated their clinical utility in many trials, and non-inferiority to anterior cervical discectomy and fusion (ACDF) is well established (1-25). Studies also show maintenance of disc space height and motion at the operated level. Additionally, secondary surgeries occur less after TDR than ACDF (6,26,27).
However, articulated designs have biomechanical limitations. None have the shock absorption provided by a viscoelastic polymer. The non-constrained or semi-constrained designs do not deliver the stability provided by a healthy disc, and lead to overloading of posterior elements (28). There is a good deal of analytical and clinical evidence suggesting that long-term implantation of articulating devices places the facets under abnormal and excessive loading, creating an environment for facet degeneration and reoccurrence of localized pain (29-33). Some metal-on-plastic devices have been shown to deform, fail or become impinged on one area of the core and move at only one or neither of the two articulating surfaces (34,35). Patients with metal on metal devices demonstrate increased levels of the metallic ions from the metals used to manufacture them. Although these articulating devices restore motion to the spinal segment, it is not natural motion. The lack of viscoelasticity necessary to replicate the shock absorbing function of the native disc has potentially negative effects such as facet degeneration, failure to relieve pain and diminish disability, a resultant need for revision surgery, and adjacent level degeneration.
The healthy human disc is viscoelastic and has six degrees of freedom. The natural disc provides for tri-planar (three-dimensional) motion: flexion and extension (sagittal plane); lateral bending (frontal plane); rotation, and compression (axial plane). The viscoelastic nature allows for variation in the degree of stiffness with the frequency of any load, and is compliant under loading (shock absorber).
There are several discs which incorporate a viscoelastic component. The Bryan (Medtronic) and Advent (Orthofix) discs are metal-on-elastomer ball-and-socket articulating designs. The M6 (Spinal Kinetics) is a non-articulating, viscoelastic device but is not securely bonded (constrained).
It has been proposed that an elastomeric one-piece intervertebral prosthesis might be the most physiological implant for mimicking physiologic levels of shock absorption and flexural stiffness (36). Currently, only a few cervical TDRs fit this design description. The CAdisc-C (Rainier) is a graduated-modulus one-piece elastomeric device. The CP-ESP (FH Orthopedics) cervical disc consists of titanium plates securely fixed to a two-part urethane core. This disc has been implanted in Europe since 2012. One published study of 2-year follow-up of 62 patients at one or two levels reported good clinical outcomes and performance of the device (36).
The Freedom® Cervical Disc (FCD, AxioMed LLC), presented in this study, is a one-piece viscoelastic artificial disc consisting of an elastomeric core bonded to titanium alloy retaining plates which is intended to re-establish the function of the cervical spinal segment, augmenting the existing anatomical structures. Function is established by: establishing flexibility and natural resistance while creating stability within the functional spinal unit (FSU); providing viscoelasticity to mimic the dynamic stiffness and load sharing in the natural disc; preserving physiological range of motion (ROM) in flexion, extension, lateral bending, rotation, and compression; and, providing the correct spine alignment and lordosis. The objective of this study is to present clinical outcome data from a 2-year post-market study of the FCD in Europe.
Methods
Thirty-nine patients were enrolled by five study sites according to the criteria in Table 1 between February 2013 and March 2014. Subjects received one- or two-level cervical TDR (FCD, AxioMed LLC, Figure 1) and underwent follow-up for two years. The study received local ethics approval for each study center, and all subjects gave written consent prior to study enrollment [Clinical trial registration No. NCT01763619 (clinicaltrials.gov)]. The ethics committees and approval information is included in Table 2.

Full table

Full table
Clinical outcomes included improvement of neck disability index (NDI) and visual analog scale (VAS) scores regarding the severity and frequency of neck and arm pain from baseline to 2-year follow-up, neurological examinations (manual muscle test, sensory and reflex assessments), the patient’s view on the success of surgery, complications, and subsequent surgical interventions. The NDI was used according to the original publication by Vernon et al. (37). The score obtained was multiplied by two to produce a percentage score.
Implant and surgery
The FCD is a viscoelastic device intended to replace symptomatic degenerative cervical discs. The device functions by restoring the natural flexibility (motion) and stiffness (load carrying capacity) of the spinal system. This device re-establishes the local spinal segment, augmenting the existing anatomical structures (facets, muscles and ligaments) that make-up the FSU through its unique design and material make-up. The FCD is indicated for use only if conservative care (approximately 6 weeks of non-operative care) fails to reduce symptoms. The FCD is implanted via an anterior open procedure.
The device consists of an elastomeric polymer core, two retaining plates, and one end cap that engages the superior retaining plate. The polymer core consists of a silicone polycarbonate urethane copolymer (trade name: CarboSil® TSPU) molded between two titanium alloy (ISO 5832-2, and ISO 5832-3) retaining plates which are porous bead coated. The device is offered in one wedge angle (8°), six sizes configured with three superior plate sizes ranging from 13 mm × 16 mm to 17 mm × 20 mm, three inferior plate sizes ranging from 12 mm × 15 mm to 16 mm × 19 mm and axial heights ranging from 5.7 to 6.9 mm. The FCD is supplied with surgical instrumentation which facilitates implantation.
Surgery was performed by a total of five surgeons in five centers. For detailed information on case distribution by center and surgeon see Table 2. Following a standard anterior approach and after total anterior discectomy and decompression the implant bed was prepared, and the implant size and height was tested by trial components. The preparation of implant rail slots into the caudal and cephalad vertebral bodies, as well as the implant’s one-piece design, allows en bloc implantation, avoiding over-distraction.
Postoperative care was handled according to the center’s standard procedures. The physical initial and follow-up examinations were done by either the investigators or other surgeons at the hospital.
Results
Patient demographics
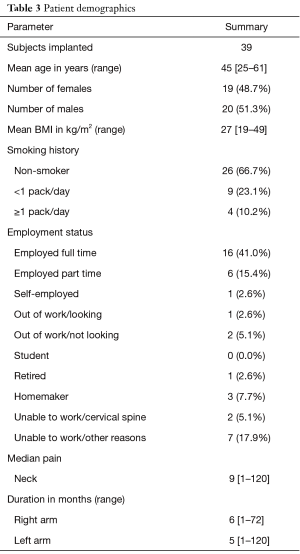
A total of 39 patients from five locations in Germany and Switzerland were enrolled in the study (Table 2). The study population (Table 3) represents a similar distribution of male 20 (51%) and female 19 (49%) subjects with an average age of 45 years.
Full table
Intraoperative details and discharge
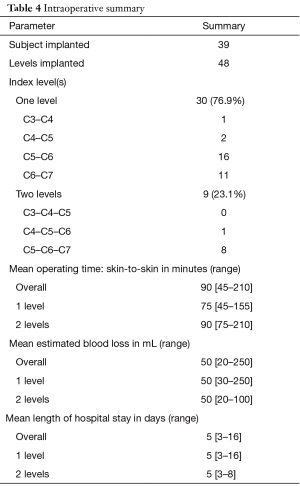
Thirty FCD were implanted at a single level between C3 and C7, and nine [9] were implanted at two adjacent levels between C4 and C7 (Table 4). Based on surgical expertise, 50% of the surgeries was completed in 90 min, with an average blood loss of 50 mL, and operating time ranged from 45 to 210 min. For all patients in the study, intra-operative and post-op periods were uneventful, and no complications due to procedure or implant were observed.
Full table
Clinical outcome
No device related adverse events were identified by the principle investigators. Three [3] serious adverse events were related to the procedure. One, reported for retropharyngeal hematoma, was definitely procedure related. The remaining two serious adverse events, paresthesia of finger and dysphagia, were reported to be possibly related to the procedure. None of the procedure related adverse events were associated with malfunction of the surgical instruments. No reported adverse event was categorized as serious requiring vigilance reporting to local regulatory authorities. No devices were explanted during the study. X-rays illustrative of one- and two-level prostheses in flexion and extension are shown in Figures 2 and 3.
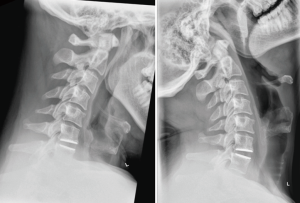
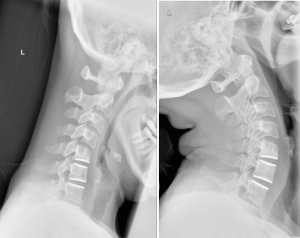
All patient self-administered clinical outcome measures showed continuous clinical significance from pre-operative evaluation and over the 2-year follow-up.
Preoperatively, 62% of participants had sensory deficit, 21% had reflex deficit, and 38% had motor function deficit. These values improved tremendously to 32%, 11% and 16% for sensory, reflex and motor functions, respectively, by the time of discharge and continued to improve. At 2 years follow-up, sensory, reflex and motor function deficits persisted in only 17%, 6% and 3% of patients, respectively (Tables 5-7).
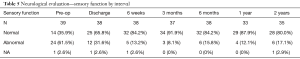
Full table
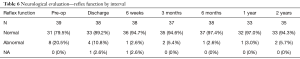
Full table
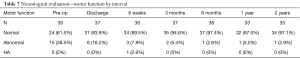
Full table
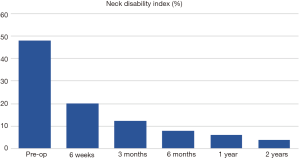
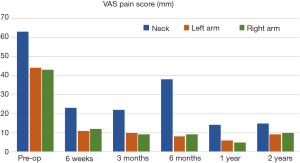
Post operation, 50% of the patients were discharged 5 days after the surgery. Significant improvement was observed immediately at discharge. Change in preoperative NDI and VAS mean scores demonstrated diminished disability and pain with improved functional status. Pre-operative NDI improved from an average of 48% to 4% at 2 years (Table 8 and Figure 4). Neck pain intensity decreased progressively from a preoperative value of 63 to 15 mm 2 years. Right and left arm pain intensity showed a similar decline in value, from 43 to 10 mm and 44 to 9 mm, respectively, during similar follow-up period (Table 9 and Figure 5). Neck, right arm and left arm pain frequency declined steadily throughout the two-year follow up period (Table 10).

Full table
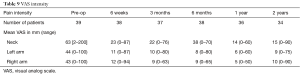
Full table

Full table
Patient satisfaction was high, with 83% of patients responding that they would “definitely” choose to have the same treatment for their neck/arm condition and another 11% responding that they would “probably” choose to have the same treatment. None of the patients responded that they would ‘definitely not’ have the same treatment.
Discussion
The FCD is one of very few TDRs with a viscoelastic, one-piece design that is intended to provide stability, flexibility, load sharing, natural resistance, alignment and lordosis. While it has been theorized that this design type will function most like the healthy intervertebral disc, it remains to be proven clinically. Will this type of design be the most successful at protecting the surrounding anatomy by providing stability and resisting excess motion? Will it slow the degenerative process because it restores proper alignment and lordosis? These questions remain to be answered by long term studies of one-piece viscoelastic TDRs.
In this study of one- and two-level implantation of a one-piece, viscoelastic TDR, all outcome measures showed significant, clinically important improvements from baseline to follow-up at 2 years. While all patients had sensory and/or motor deficits preoperatively, 77% of patients were asymptomatic at two years follow-up. Patient satisfaction was also very high.
FCD patients experienced improvements in neck and arm pain similar to those seen for both arthroplasty and fusion patients in randomized (4,6,8,9,13,38-40) and non-randomized studies (36,41-43). VAS neck pain for the FCD was reduced to 15 mm at 2 years compared to 24 and 38 mm for the M6 (41,42) in two different studies, and was reduced to 14 mm at 1 year compared to 26.5 mm for the CP ESP. The VAS neck pain of 15 mm reported in this study is similar to reported ranges of 13 to 27 mm for articulating discs including ProDisc-C, Mobi-C, Secure C, Discover, Bryan, activC, Kineflex C and Prestige, and 16 to 26 mm for the fusion controls from the same studies (1,4,19,25,38-40,43). VAS arm pain for the FCD was reduced to 9 mm (left) and 10 mm (right) at 2 years, compared to 21/16 mm (right/left) and 39 mm for the M6 (41,42), and was reduced to 7 mm at 1 year compared to 24 mm for the CP ESP. VAS arm pain of 9 and 10 mm at 2 years follow-up is similar to reported ranges of 7 to 14 mm for articulating discs and 8 to 19 mm for ACDF (1,19,25,38,43).
NDI for the FCD compared favorably to that for other TDRs and fusion. NDI was 4% at 2 years follow-up, compared to 20.8% (41) and 27.9% (42) for the M6 cervical disc in two studies. NDI at 2 years follow-up ranged from 12% to 40% for studies involving articulating discs (listed above), and from 17% to 38% in the fusion control groups for those studies (1,4,19,25,38-40,43). NDI was reported to be 24% for the CP ESP disc at 1 year follow-up (36), compared to an NDI of 6% for the FCD of at 1 year follow-up.
These results indicate that the technology is performing well for the follow-up period of 2 years. Additional studies and long-term patient follow-up are still needed to assess long-term clinical outcomes of viscoelastic one-piece cervical TDR in general, and this technology in particular.
Conclusions
The FCD performs as expected in patients with single- and two-level degenerative disc disease. In this early clinical experience with the FCD, patients experienced similar pain relief and lower disability at 2 years follow-up compared to both articulating and viscoelastic TDRs.
Acknowledgements
The authors thank RQMIS Inc. for managing the study data and contributing their expertise to the manuscript.
The study was funded by AxioMed LLC. AxioMed research staff were involved in the collation and writing of the manuscript.
Footnote
Conflicts of Interest: The authors have no conflict of interest to declare.
Ethical statement: The study received local ethics approval for each study center, and all subjects gave written consent prior to study enrollment [Clinical trial registration No. NCT01763619 (clinicaltrials.gov)].
References
- Burkus JK, Traynelis VC, Haid RW Jr, et al. Clinical and Radiographic Analysis of an Artificial Cervical Disc: 7-Year Follow-Up from the Prestige Prospective Randomized Controlled Clinical Trial. J Neurosurg Spine 2014;21:516-28. [Crossref] [PubMed]
- Caruso R, Pesce A, Marrocco L, et al. Anterior Approach to the Cervical Spine for Treatment of Spondylosis or Disc Herniation: Long-Term Results. Comparison Between ACD, ACDF, TDR. Clin Ter 2014;165:e263-70. [PubMed]
- Cincu R, Lorente FA, Gomez J, et al. Long Term Preservation of Motion with Artificial Cervical Disc Implants: A Comparison Between Cervical Disc Replacement and Rigid Fusion with Cage. Asian J Neurosurg 2014;9:213-7. [PubMed]
- Coric D, Nunley PD, Guyer RD, et al. Prospective, Randomized, Multicenter Study of Cervical Arthroplasty: 269 Patients from the Kineflex C Artificial Disc Investigational Device Exemption Study with a Minimum 2-Year Follow-Up. J Neurosurg Spine 2011;15:348-58. [Crossref] [PubMed]
- Davis RJ, Nunley PD, Kim KD, et al. Two-Level Total Disc Replacement with Mobi-C Cervical Artificial Disc Versus Anterior Discectomy and Fusion: A Prospective, Randomized, Controlled Multicenter Clinical Trial with 4-Year Follow-Up Results. J Neurosurg Spine 2015;22:15-25. [Crossref] [PubMed]
- Delamarter RB, Murrey D, Janssen ME, et al. Results at 24 Months from a Prospective, Randomized, Multicenter Investigational Device Exemption Trial of ProDisc-C Versus Anterior Cervical Discectomy and Fusion with 4-Year Follow-up and Continued Access Patients. SAS J 2010;4:122-8. [Crossref] [PubMed]
- Gao Y, Liu M, Li T, et al. A Meta-Analysis Comparing the Results of Cervical Disc Arthroplasty with Anterior Cervical Discectomy and Fusion (ACDF) for the Treatment of Symptomatic Cervical Disc Disease. J Bone Joint Surg Am 2013;95:555-61. [Crossref] [PubMed]
- Gornet MF, Lanman TH, Burkus JK, et al. Cervical Disc Arthroplasty with the Prestige LP Disc Versus Anterior Cervical Discectomy and Fusion, at 2 Levels: Results of a Prospective, Multicenter Randomized Controlled Clinical Trial at 24 Months. J Neurosurg Spine 2017;26:653-67. [Crossref] [PubMed]
- Hisey MS, Zigler JE, Jackson R, et al. Prospective, Randomized Comparison of One-Level Mobi-C Cervical Total Disc Replacement vs. Anterior Cervical Discectomy and Fusion: Results at 5-Year Follow-Up. Int J Spine Surg 2016;10:10. [Crossref] [PubMed]
- Lanman TH, Burkus K, Dryer RG, et al. Long-Term Clinical and Radiographic Outcomes of the Prestige LP Artificial Cervical Disc Replacement at 2 Levels: Results from a Prospective Randomized Controlled Clinical Trial. J Neurosurg Spine 2017;27:7-19. [Crossref] [PubMed]
- Luo J, Huang S, Gong M, et al. Comparison of Artificial Cervical Arthroplasty Versus Anterior Cervical Discectomy and Fusion for One-Level Cervical Degenerative Disc Disease: A Meta-Analysis of Randomized Controlled Trials. Eur J Orthop Surg Traumatol 2015;25:S115-25. [Crossref] [PubMed]
- Malham GM, Parker RM, Ellis NJ, et al. Cervical Artificial Disc Replacement with ProDisc-C: Clinical and Radiographic Outcomes with Long-Term Follow-Up. J Clin Neurosci 2014;21:949-53. [Crossref] [PubMed]
- Murrey D, Janssen M, Delamarter R, et al. Results of the prospective, randomized, controlled multicenter Food and Drug Administration investigational device exemption study of the ProDisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. Spine J 2009;9:275-86. [Crossref] [PubMed]
- Nandyala SV, Marquez-Lara A, Fineberg SJ, et al. Comparison Between Cervical Total Disc Replacement and Anterior Cervical Discectomy and Fusion of 1 to 2 Levels from 2002 to 2009. Spine 2014;39:53-7. [Crossref] [PubMed]
- Pandey PK, Pawar I, Gupta J, et al. Comparison of Outcomes of Single-Level Anterior Cervical Discectomy with Fusion and Single-Level Artificial Cervical Disc Replacement for Single-Level Cervical Degenerative Disc Disease. Spine 2017;42:E41-9. [Crossref] [PubMed]
- Phillips FM, Lee JY, Geisler FH, et al. A Prospective, Randomized, Controlled Clinical Investigation Comparing PCM Cervical Disc Arthroplasty with Anterior Cervical Discectomy and Fusion; 2-Year Results from the US FDA IDE Clinical Trial. Spine 2013;38:E907-18. [Crossref] [PubMed]
- Rao MJ, Nie SP, Xiao BW, et al. Cervical Disc Arthroplasty Versus Anterior Cervical Discectomy and Fusion for Treatment of Symptomatic Cervical Disc Disease: A Meta-Analysis of Randomized Controlled Trials. Arch Orthop Trauma Surg 2015;135:19-28. [Crossref] [PubMed]
- Upadhyaya CD, Wu JC, Trost G, et al. Analysis of the Three United States Food and Drug Administration Investigational Device Exemption Cervical Arthroplasty Trials. J Neurosurg Spine 2012;16:216-28. [Crossref] [PubMed]
- Vaccaro A, Beutler W, Peppelman W, et al. Clinical Outcomes with Selectively Constrained Secure-C Cervical Disc Arthroplasty; Two-Year Results from a Prospective, Randomized, Controlled, Multicenter Investigational Device Exemption Study. Spine 2013;38:2227-39. [Crossref] [PubMed]
- Wu AM, Xu H, Mullinix KP, et al. Minimum 4-Year Outcomes of Cervical Total Disc Arthroplasty Versus Fusion; A Meta-Analysis Based on Prospective Randomized Controlled Trials. Medicine (Baltimore) 2015;94:e665. [Crossref] [PubMed]
- Xu B, Ma JX, Tian JH, et al. Indirect Meta-Analysis Comparing Clinical Outcomes of Total Cervical Disc Replacements with Fusions for Cervical Degenerative Disc Disease. Sci Rep 2017;7:1740. [Crossref] [PubMed]
- Yao Q, Liang F, Xia Y, et al. A Meta-Analysis Comparing Total Disc Arthroplasty with Anterior Cervical Discectomy and Fusion for the Treatment of Cervical Degenerative Diseases. Arch Orthop Trauma Surg 2016;136:297-304. [Crossref] [PubMed]
- Yin S, Yu X, Zhou S, et al. Is Cervical Disc Arthroplasty Superior to Fusion for Treatment of Symptomatic Cervical Disc Disease? A Meta-Analysis. Clin Orthop Relat Res 2013;471:1904-19. [Crossref] [PubMed]
- Yu L, Song Y, Yang S, et al. Systematic Review and Meta-Analysis of Randomized Controlled Trials: Comparison of Total Disk Replacement With Anterior Cervical Decompression and Fusion. Orthopedics 2011;34:e651-8. [PubMed]
- Zigler JE, Delamarter R, Murrey D, et al. ProDisc-C and Anterior Cervical Discectomy and Fusion as Surgical Treatment for Single-Level Cervical Symptomatic Degenerative Disc Disease. Spine 2013;38:203-9. [Crossref] [PubMed]
- Chen C, Zhang X, Ma X. Durability of Cervical Disc Arthroplasties and Its Influence Factors; A Systematic Review and a Network Meta-analysis. Medicine (Baltimore) 2017;96:e5947. [Crossref] [PubMed]
- Garrido BJ, Taha TA, Sasso RC. Clinical Outcomes of Bryan Cervical Disc Arthroplasty; A Prospective, Randomized, Controlled, Single Site Trial With 48-Month Follow-up. J Spinal Disord Tech 2010;23:367-71. [Crossref] [PubMed]
- Lemaire JP, Skalli W, Lavaste F, et al. Intervertebral Disc Prosthesis: Results and Prospects for the Year 2000. Clin Orthop Relat Res 1997.64-76. [Crossref] [PubMed]
- Denozière G, Ku DN. Biomechanical Comparison between Fusion of the Two Vertebrae and Implantation of an Artificial Intervertebral Disc. J Biomech 2006;39:766-75. [Crossref] [PubMed]
- Park CK, Ryu KS, Jee WH. Degenerative Changes of Discs and Facet Joints in Lumbar Total Disc Replacement Using ProDisc II: Minimum Two-Year Follow-Up. Spine 2008;33:1755-61. [Crossref] [PubMed]
- Phillips FM, Diaz R, Pimenta L. The Fate of the Facet Joints after Lumbar Total Disc Replacement: a Clinical and MRI Study. Spine J 2005;5:75S. [Crossref]
- Punt IM, Visser VM, van Rhijn LW, et al. Complications and Reoperations of the SB Charite Lumbar Disc Prosthesis: Experience in 75 Patients. Eur Spine J 2008;17:36-43. [Crossref] [PubMed]
- van Ooij A, Oner FC, Verbout AJ. Complications of Artificial Disc Replacement: A Report of 27 Patients with the SB Charite Disc. J Spinal Disord Tech 2003;16:369-83. [Crossref] [PubMed]
- Lebl DR, Cammisa FP Jr, Girardi FP, et al. The Mechanical Performance of Cervical Total Disc Replacements In Vivo: Prospective Retrieval Analysis of ProDisc-C Devices. Spine 2012;37:2151-60. [Crossref] [PubMed]
- Wu W, Lyu J, Liu H, et al. Wear Assessments of a New Cervical Spinal Disk Prosthesis: Influence of Loading and Kinematic Patterns During In Vitro Wear Simulation. Proc Inst Mech Eng H 2015;229:619-28. [Crossref] [PubMed]
- Lazennec JY, Aaron A, Ricart O, et al. The Innovative Viscoelastic CP ESP Cervical Disk Prosthesis With Six Degrees of Freedom: Biomechanical Concepts, Development Program and Preliminary Clinical Experience. Eur J Orthop Surg Traumatol 2016;26:9-19. [Crossref] [PubMed]
- Vernon H, Mior S. The Neck Disability Index: A Study of Reliability and Validity. J Manipulative Physiol Ther 1991;14:409-15. [PubMed]
- Skeppholm M, Lindgren L, Henriques T, et al. The Discover Artificial Disc Replacement Versus Fusion in Cervical Radiculopathy – A Randomized Controlled Outcome Trial with 2-Year Follow-Up. Spine J 2015;15:1284-94. [Crossref] [PubMed]
- Zhang HX, Shao YD, Chen Y, et al. A Prospective, Randomised, Controlled Multicentre Study Comparing Cervical Disc Replacement with Anterior Cervical Decompression and Fusion. Int Orthop 2014;38:2533-41. [Crossref] [PubMed]
- Sasso RC, Anderson PA, Riew KD, et al. Results of Cervical Arthroplasty Compared with Anterior Discectomy and Fusion: Four-Year Clinical Outcomes in a Prospective, Randomized, Controlled Trial. J Bone Joint Surg Am 2011;93:1684-92. [Crossref] [PubMed]
- Lauryssen C, Coric D, Dimmig T, et al. Cervical Total Disc Replacement Using a Novel Compressible Prosthesis: Results from a Prospective Food and Drug Administration-Regulated Feasibility Study with 24-Month Follow-Up. Int J Spine Surg 2012;6:71-7. [Crossref] [PubMed]
- Reyes-Sanchez A, Miramontes V, Olivarez LM, et al. Initial Clinical Experience with a Next Generation Artificial Disc for the Treatment of Symptomatic Degenerative Cervical Radiculopathy. SAS J 2010;4:9-15. [Crossref] [PubMed]
- Meisel HJ, Jurak L, Antinheimo J, et al. Four-Year Results of a Prospective Single-Arm Study on 200 Semi-Constrained Total Cervical Disc Prostheses: Clinical and Radiographic Outcome. J Neurosurg Spine 2016;25:556-65. [Crossref] [PubMed]