Multifocal intradural extramedullary anaplastic ependymoma of the spine
Introduction
Ependymomas are neuroectodermal tumours arising from the ependymal lining of the ventricles and the central canal of the spinal cord (1). They are the most common intramedullary spinal cord tumour in adults (83%) (2,3). They are classified as either WHO grade II (classical) or grade III (anaplastic) (2). They are however very rarely found in an intradural extramedullary (IDEM) location, with only 33 cases reported. Multifocal IDEM are even more rare with only 5 of the 33 cases having multiple lesions. Only 5 of the 33 cases were anaplastic tumours. Only one previous case was multifocal and anaplastic (4).
We present the second case of multifocal IDEM anaplastic (grade III) ependymoma.
Case presentation
History & examination
A 47-year old female dental nurse of Argentinian origin presented with left sacral and thigh pain and paraesthesia radiating to her groin. She had no neurological deficit. Past medical history included treated tuberculosis and iron-deficiency anaemia.
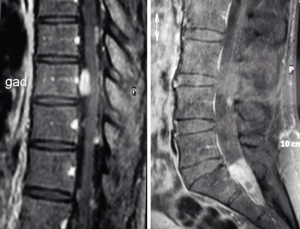
Magnetic resonance imaging (MRI) revealed a 20mm complex intradural, extramedullary lesion at the level of the second sacral segment (Figure 1). There were multiple (>10) other intradural, extramedullary spinal lesions extending from the cervicomedullary junction to the lumbar region. The largest, at T9, measured 12 mm × 13 mm. The lesions were isointense to brain on T1 and T2-weighted sequences, with uniform contrast enhancement in some lesions, and peripheral enhancement in other lesions. There were no intracranial lesions.
Operation
The patient underwent a sacral laminectomy. A 2-cm soft grey-pink tumour was resected from within the cauda equine, with two smaller lesions (<3 mm) arising from the sacral nerve roots. All nerve roots were preserved. Postoperatively, the patient experienced mild urinary retention, which resolved after 2 weeks. All three lesions were anaplastic ependymomas (WHO grade III).
The three tumours had a similar appearance. They were composed of cells with small to intermediate sized, mildly pleomorphic, nuclei and moderate amounts of eosinophilic cytoplasm. Numerous perivascular pseudorosettes and occasional true ependymal rosettes were present. The cellularity of the lesions varied from moderate to high. In the more cellular areas the mitotic index was up to 14 per high power field. The Ki67 index was up to 30%. Tumour cells were positive for GFAP, S100 vimentin, EMA and CD99 and negative for neurofilament protein. Focal paranuclear dot-like staining was noted with EMA and CD99.
Post-operative course
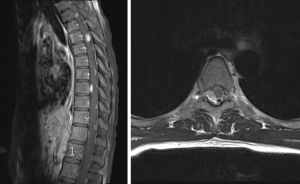
Post-operative MRI confirmed complete resection of the three lesions (Figure 2). No other treatment was performed at this point. One year later surveillance MRI showed growth of the lesion at T5 lying on the posterior aspect of the cord. All other lesions were stable. The patient underwent surgery with a thoracic laminectomy from T4–6. Resection of the lesion as well as two adjacent smaller lesions was performed. The T5 tumour was also an anaplastic ependymoma (WHO grade III), the smaller nodules were histologically classical ependymoma (WHO grade II). Postoperatively the patient had mild sensory disturbance over the right side of the trunk, which resolved over three months.
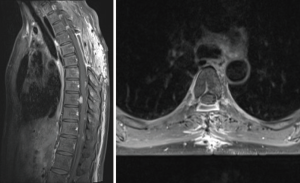
One month later, the patient underwent craniospinal proton beam therapy at 46 Gray. There were no immediate adverse neurological effects and she returned to work. The lesions were stable on MRI (Figure 3).
At last follow up, 4 years after presentation, the patient remains asymptomatic and neurologically intact. She continues full time employment. Radiographically all lesions are stable. Routine surveillance MRI continues every 6 months.
Discussion
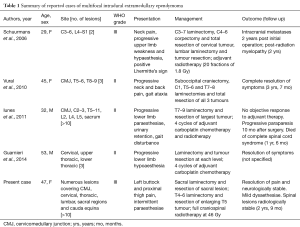
Non-myxopapillary IDEM ependymomas are very rare tumours with only 33 cases reported (25 female, 7 male). Only five cases were multifocal (Table 1), and only one of these was anaplastic (1,4-6). The most common site for IDEM ependymomas is the thoracic spine (n=16), followed by cervical (n=8) and lumbosacral (n=4). The remaining cases are multifocal (1,4-6). Our case is very unusual in that the lesions were both multifocal and anaplastic at presentation. Only one such case has been reported previously.
Full table
Most cases of grade II tumours have been treated with surgical intervention only. More aggressive (grade III) lesions have been given differing regimens of adjuvant radiotherapy with variable success (4,7-13). Guppy et al. (7) used external beam radiation with total dose of 5000cGy for grade III disease. Three cases describe using 28 fractions of 1.8 Gy for a total dose of 50.4 Gy, one for a grade II lesion (9,10,13). used 20 sessions of 1.8 Gy, with cervical and lumbar boost to a total dose of 54 Gy for multifocal anaplastic disease. Severino et al. (11) described the only case of IDEM ependymoma in a child (grade II), using craniospinal irradiation of a total of 3,600 cGy for a grade II lesion. Details of radiotherapy were not provided in two cases (8,12).
Clinical presentation may be with radicular pain and dysaesthesiae, but depends on the location of tumour (1,5,7).
Of the five previously reported patients with anaplastic ependymoma, four had solitary lesions (three thoracic, one cervical) and one had multiple lesions (7-9,12,14). One recurred following partial resection and radiotherapy (28 fractions of 1.8 Gy), 14 months following surgery. The patient refused further therapy (9). Cerase et al. (12) also describe recurrence following radiotherapy with worsening clinical status; the patient died 1 month after surgical resection. The remaining 2 cases of solitary anaplastic lesions described partial or full recovery at latest follow-up after surgical resection and adjuvant radiotherapy (7,8,14). Guppy et al. (7) describe stable residual disease following total external beam radiotherapy (EBRT) dose of 5,000 cGy, however follow-up was only 6 months. Kinsman et al. (8) did not provide a follow-up period at all, however claim neurological improvement following unspecified adjuvant radiotherapy and surgery.
Schuurmans et al. (4) reported a case of multifocal anaplastic ependymoma in a 29-year old female with multiple lesions. This patient developed intracranial metastases and post-radiation myelopathy two years following treatment. Four years after diagnosis our patient is neurologically intact. There are no cranial lesions and her spinal lesions are stable.
IDEM ependymomas are thought to arise from ectopic ependymal cells derived from remnants of the neural tube (5,10,13). However, their pathogenesis remains ill-defined, with recent evidence suggesting glial stem cells may be the cell of origin (15). It is important to note that malignant features do not necessarily correlate with poor prognosis (16). This is demonstrated in our case, where four of the six resected lesions were grade III, and two of the six resected lesions grade II lesions. Our patient is neurologically intact and independent 4 years after initial diagnosis. This is in contrast to the case of multifocal grade II disease described by Iunes et al. (5), with the patient dying 18 months after diagnosis despite surgery and adjuvant chemoradiotherapy. Future studies of molecular characteristics of ependymoma may facilitate more precise prognosis (2).
Treatment recommendations for multifocal IDEM anaplastic ependymoma are difficult due to their rarity. The goal of surgery is maximal resection with preservation of neurological function. Radiotherapy has been used in our case. Surgical decompression and whole spine radiotherapy was used by Schuurmans et al. (4) in their case, however cranial metastases developed two years following therapy. Treatment for multifocal grade II ependymoma may include surgical resection with adjuvant radiotherapy and chemotherapy reserved for incomplete resection or recurrent disease (1,5,6). Vural et al. (6) found surgical resection alone adequate in their case of a grade II ependymoma. Guarnieri et al. (1) used surgical resection and four cycles of carboplatin, however follow up period and choice of chemotherapy was not specified (1). Iunes et al. (5) reported a case of multifocal grade II tumour with multiple lesions and a tumour burden comparable to ours (>10 separate lesions). The patient was treated with four cycles of carboplatin for 4 weeks and radiotherapy of the whole brain (39.5 Gy) and neuraxis (36 Gy). The patient progressed to complete paraplegia 10 months following surgery and died of complete spinal cord syndrome at one year. Identification of prognostic factors other than tumour grade will be important to guide treatment. In our case, adjuvant radiotherapy was used, however no chemotherapy has been used to date.
Conclusions
We report the second case of multifocal anaplastic ependymoma of the spinal cord arising in a 46-year old female. Treatment so far has involved surgical resection of six separate lesions and full craniospinal irradiation using proton beam therapy. All remaining lesions are currently stable and she is neurologically intact at 48-month follow up.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Guarnieri G, Tecame M, Izzo R, et al. Multisegmental diffuse intradural extramedullary ependymoma an extremely rare case. Neuroradiol J 2014;27:179-85. [Crossref] [PubMed]
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803-20. [Crossref] [PubMed]
- Shrivastava RK, Epstein FJ, Perin NI, et al. Intramedullary spinal cord tumors in patients older than 50 years of age: management and outcome analysis. J Neurosurg Spine 2005;2:249-55. [Crossref] [PubMed]
- Schuurmans M, Vanneste JA, Verstegen MJ, et al. Spinal extramedullary anaplastic ependymoma with spinal and intracranial metastases. J Neurooncol 2006;79:57-9. [Crossref] [PubMed]
- Iunes EA, Stávale JN, de Cássia Caldas Pessoa R, et al. Multifocal intradural extramedullary ependymoma: Case report. J Neurosurg Spine 2011;14:65-70. [Crossref] [PubMed]
- Vural M, Arslantas A, Ciftci E, et al. Multiple intradural-extramedullary ependymomas: proven dissemination by genetic analysis. J Neurosurg Spine 2010;12:467-73. [Crossref] [PubMed]
- Guppy KH, Hou L, Moes GS, et al. Spinal intradural, extramedullary anaplastic ependymoma with an extradural component: Case report and review of the literature. Surg Neurol Int 2011;2:119. [Crossref] [PubMed]
- Kinsman MJ, Callahan JD, Hattab EM, et al. Extramedullary spinal ependymoma: a diagnostic challenge and review of the literature. Clin Neurol Neurosurg 2011;113:661-4. [Crossref] [PubMed]
- Kim BS, Kim SW, Kwak KW, et al. Extra and intramedullary anaplastic ependymoma in thoracic spinal cord. Korean J Spine 2013;10:177-80. [Crossref] [PubMed]
- Moriwaki T, Iwatsuki K, Ohnishi Y, et al. Intradural extramedullary spinal ependymoma: A case report of malignant transformation occurring. Asian Spine J 2013;7:139-42. [Crossref] [PubMed]
- Severino M, Consales A, Doglio M, et al. Intradural extramedullary ependymoma with leptomeningeal dissemination: the first case report in a child and literature review. World Neurosurg 2015;84:865.e13-9. [Crossref] [PubMed]
- Cerase A, Venturi C, Oliveri G, et al. Intradural extramedullary spinal anaplastic ependymoma. Case illustration. J Neurosurg Spine 2006;5:476. [Crossref] [PubMed]
- Son DW, Song GS, Han IH, et al. Primary extramedullary ependymoma of the cervical spine: Case report and review of the literature. J Korean Neurosurg Soc 2011;50:57-9. [Crossref] [PubMed]
- Oliver B, de Castro A, Sarmiento MA, et al. Dorsal extramedullary ependymoma (author's transl). Arch Neurobiol (Madr) 1981;44:215-24. [PubMed]
- Karsy M, Guan J, Sivakumar W, et al. The genetic basis of intradural spinal tumors and its impact on clinical treatment. Neurosurgical Focus 2015;39:E3. [Crossref] [PubMed]
- Ross GW, Rubinstein LJ. Lack of histopathological correlation of malignant ependymomas with postoperative survival. J Neurosurg 1989;70:31-6. [Crossref] [PubMed]


