Posterolateral thoracic decompression with anterior column cage reconstruction versus decompression alone for spinal metastases with cord compression: analysis of perioperative complications and outcomes
Introduction
Surgical management of spinal metastases remains a palliative undertaking (1). The goals of surgery are aimed at improving the overall quality of life. Additionally, surgical resection serves to stabilize and improve neurological function, prevent local progression of tumor and provide pain relief (1). Although the vast majority of patients with spinal metastases ultimately succumb to complications or progression of their primary disease process, good functional and neurological outcomes have been reported with surgical treatment (2,3). Traditionally, most surgeons favored the anterior transthoracic approach to directly address ventral metastatic involvement (3-8). The abandonment of posterior laminectomy for assessing ventral spinal cord led to the development of posterolateral approaches such as the transpedicular (TP), costotransversectomy (CT) and lateral extracavitary approaches (9-14). While a few studies have reported favorable outcomes with posterolateral approaches, the morbidity and mortality still remain high with some studies reporting mortality rates of 6% to 11% (9-11,13-17). The reasons behind this high incidence of perioperative morbidity and mortality have not been fully elucidated but most likely multifactorial (9,12-14). In this study, we attempt to investigate whether the addition of anterior column reconstruction to a posterolateral approach in the surgical management of spinal metastases increases operative and perioperative morbidity and mortality.
Methods
A retrospective review of the medical records of all adult (age >18 years) patients who had surgical resection for spinal metastases between January 2000 and December 2008 at our institution was performed. Patients who underwent anterior transthoracic approaches and minimally invasive approaches were excluded. Clinical, radiographic, operative and pathologic reports were reviewed. The Charlson comorbidity index was used to classify each patient’s comorbidities (18). We defined the preoperative and postoperative functional neurological status using the American Spinal Injury Association (ASIA) impairment scale (Table 1). Each patient’s neurological status at their last follow-up encounter was assessed using the ASIA scale.

Full table
Operative technique
CT
The CT approach has been described elsewhere (19). An illustration is shown in Figure 1A. Briefly, patients were positioned prone on a Jackson table. Standard perioperative antibiotic agents were administered prior to skin incision. All pressure points were adequately padded. When appropriate, neurophysiologic monitoring was used. After a midline skin incision, bilateral subperiosteal muscle dissection was performed. The affected level was localized with fluoroscopy. Thoracic pedicle screws were placed bilaterally, usually 2 to 3 levels above and below the affected level. A laminectomy was then performed along with resection of the transverse process and the proximal 2–3 cm of adjoining rib. The pleura was then meticulously dissected off the vertebral bodies without violating the parietal pleura. The intercostal bundle was then identified and followed to identify the neural foramen. The intercostal artery and nerve root at the pathologically affected level were double ligated, and divided. Ligation of nerve root occurred proximal to the dorsal root ganglion. The ipsilateral pedicle was then removed revealing the thecal sac. The vertebrectomy was then performed using a combination of high-speed diamond drill and angled curettes. When appropriate, the same procedure was performed at the contralateral side to ensure circumferential decompression. The created defect was reconstructed using a titanium cage. The precontoured rod and cross links were then connected to the pedicle screws to complete the instrumentation. Chest tubes were not routinely inserted unless obvious violation of pleura occurred.
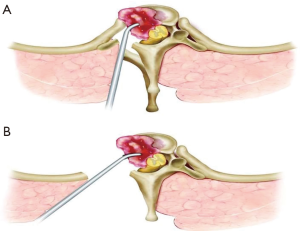
TP
A description of the TP approach has been performed elsewhere and will be described briefly (Figure 1B) (20). Patients were positioned in a prone position following induction of endotracheal anesthesia. All pressure points were adequately padded. When indicated, neurophysiologic monitoring was used. A midline incision was made followed by subperiosteal dissection to expose the posterior elements. The pathologic level was confirmed fluoroscopy. Depending on surgeon’s preference, posterior pedicle screw instrumentation was next placed followed by laminectomy, facetectomies and pedicle resections using a high-speed drill. Working around the dura, the epidural tumor was then removed. In cases of nerve root involvement, the root was double ligated and resected proximal to the dorsal root ganglion. Posterior instrumentation was then completed. In the event of any potential violation to the pleura, chest tubes were placed. The wound was then closed in successive layers.
Statistical analysis
Statistical analysis was performed using GraphPad StatMate (GraphPad Software Inc., San Diego, California). The Fischer exacts test was used to determine statistical significance among the two groups. The Student’s t-test was used to determine statistical significance among two pertinent variables of interest. The Kaplan-Meier survival curve was used to generate overall survival. Statistical significance was defined as P<0.05.
Results
Demographics
A total of 24 patients underwent a posterolateral approach for resection of spinal metastases during the review period. One minimally invasive case was excluded. As this is a study of perioperative complications, patients who were discharged to hospice or nursing home and thus did not have outpatient follow-up were included in the study. This yielded a total of 23 patients with spinal metastasis who underwent posterolateral approach with or without vertebral body resection and reconstruction (Table 2). Eleven patients underwent a single-staged CT approach with single or multilevel vertebrectomy and 12 patients underwent TP resection of ventral epidural metastases without a vertebrectomy. Both groups had posterior pedicle screw instrumentation. The mean age was 55.9 years in the CT group and 59.3 years in the TP group. The mean follow-up was 12.7 and 5.7 months in the CT and TP groups respectively.
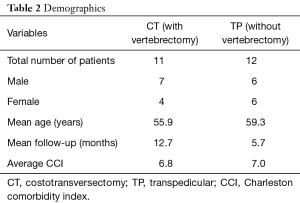
Full table
Clinical data
Pain was the most common presenting symptom in both CT (82%) and TP (83%) groups. Other presenting symptoms in CT group included weakness (45%), sensory deficits (36%) and incontinence (9%). In the TP group, 33% of patients presented with weakness, 25% with sensory deficits and 8% with incontinence. At time of surgery, 73% and 67% of patients were ambulatory in the CT and TP groups respectively. A total of 45% and 50% of patients received radiation treatment to their spinal tumors prior to surgical resection in the CT and TP groups, respectively. The indications for surgery consisted of pain, neurological deficit, spinal instability, need for histological diagnosis and tumor progression following radiation therapy. The choice of surgical approach was dictated by each treating surgeon and was not specified. The mean Charlson comorbidity index was 6.8 and 7.0 in the CT and TP groups, respectively. The clinical summaries of both groups are shown in Tables 3,4.
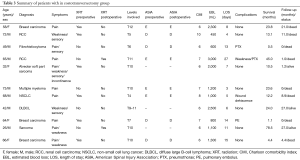
Full table
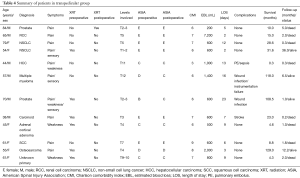
Full table
Radiographic data
Radiographic data was obtained from magnetic resonance imaging (MRI) with and without contrast from all patients. The extent of spinal tumor in the CT group consisted of two patients with solitary metastases and nine with multiple metastases. Nine patients in this group had three column involvements, 9 with >50% vertebral body height loss and 9 with >50% vertebral body involvement. Three patients within this group had tumors that involved transitional levels. All patients had radiographic spinal cord compression.
In the TP group, 12 patients had three column involvements, 3 with >50% vertebral body height loss and 5 with >50% vertebral body involvement. Three patients had tumors that involved transitional levels. All patients had radiographic spinal cord compression.
Tumor histology
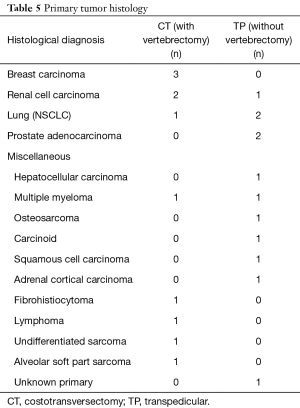
Histological diagnosis was confirmed in all cases in both groups (Table 5). In the CT group, there were 3 breast carcinomas, 2 renal cell carcinomas, 1 non-small cell lung (NSCLC), 1 alveolar soft part sarcoma, 1 fibrohistiocytoma, 1 multiple myeloma, 1 undifferentiated sarcoma, and 1 diffuse large B-cell lymphoma. Tumors in the TP group consisted of 2 NSCLC, 2 prostate carcinomas, 1 renal cell carcinoma, 1 hepatocellular carcinoma, 1 multiple myeloma, 1 carcinoid tumor, 1 adrenal cortical carcinoma, 1 osteosarcoma, 1 squamous cell carcinoma and 1 tumor of unknown primary.
Full table
Level of surgery
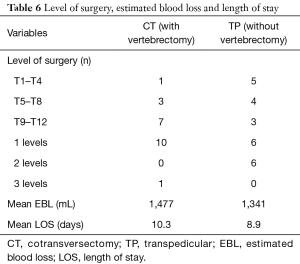
The levels of surgery were grouped into upper thoracic (T1–T4), mid-thoracic (T5–T8) and lower thoracic (T9–T12) (Table 6). The operative levels within the CT group consisted of 1, 3 and 7 upper, mid and lower thoracic, respectively. In the TP group, the levels consisted of 5, 4 and 3 upper, mid and lower thoracic, respectively. One- and 3-level vertebrectomies were performed in ten and one patients in the CT group, respectively. In the TP group, six patients underwent 1- and 2-level TP decompressions without vertebrectomies, respectively. In both groups, posterior pedicle screw supplementation was added typically at 2 levels above and below the level of vertebrectomy or decompression.
Full table
Estimated blood loss (EBL) and length of stay
The average EBL was 1,477 and 1,341 milliliters (mL) for the CT and TP groups respectively. The difference in EBL did not reach statistical significance (P=0.83). The average length of stay was 10.3 and 8.9 days in the CT and TP group, respectively (P=0.63).
Perioperative complications
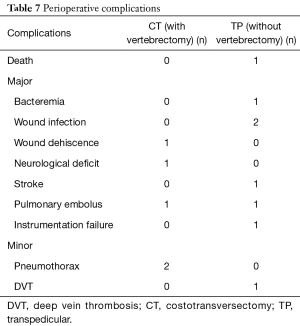
There was no intraoperative death in either group. One death attributed to sepsis occurred in the TP group. There was no perioperative death in the CT group. A total of 5 (45.5%) complications (major and minor) occurred in the CT group and 7 (58.3%) in the TP group (P=0.68) (Table 7). We observed 3 (27.3%) major (defined as any adverse event requiring additional surgical intervention or resulting in potential for long-term harm) and 2 (18.2%) minor (defined as any event not requiring additional surgical interventions, resulting in death or the potential for long-term harm) complications in the CT group. In the TP group, major complications occurred in 6 (50.0%) cases and minor complications in 1 (8.3%) patient. One patient in the CT group underwent revision surgery for wound dehiscence. Within the TP group, three patients underwent revision surgery for wound infection in 2 and instrumentation failure in 1.
Full table
Functional outcomes
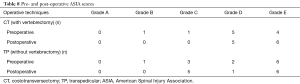
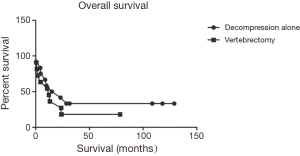
Pre- and post-operative neurological status were graded using the ASIA impairment scale (Tables 8,9). Within the CT group, 1, 1, 5 and 4 patients presented with ASIA B, C, D and E, respectively. In the TP group, 1, 3, 2 and 6 patients presented with ASIA B, C, D and E, respectively. At the time of last follow-up, in the CT group, 5 and 6 patients were graded as ASIA D and E, respectively. In the TP group, follow-up ASIA grades consisted of 5, 1 and 6 ASIA C, D and E, respectively. An improvement in ASIA grades was observed in 3 (27.3%) patients in the CT group and 1 (8.3%) in TP group. ASIA grades remained the same in 8 (72.7%) patients in CT and 10 (83.3%) patients in TP groups. No patient worsened in the CT group whereas 1 (8.3%) patient in TP group worsened. At the time of data collection, 9 (81.8%) and 8 (66.7%) patients were deceased in the CT and TP groups, respectively. The median survival was 12.2 months in the CT group and 19.0 months in the TP group. A Kaplan-Meier overall survival curve is shown in Figure 2.
Full table

Full table
Discussion
The posterolateral approaches (CT, TP and lateral extracavitary) to the ventral thoracic spine have been used for decades now with favorable results (9,21). The impetus for their development stemmed out of the need to provide an alternative to the traditional anterior transthoracic approach for treating diseases located in the ventral thoracic spine (22). Although the CT was originally developed for the treatment of Potts disease, modifications of the technique have evolved and expanded to include single and multilevel vertebral body resections with anterior cage reconstruction (22-24). The posterolateral approach offers several advantages over the anterior transthoracic routes such as the ability to perform vertebral body resections with posterior instrumentation in a single setting (20). Additionally, the need to traverse the thoracic cavity which may not be tolerated by the critically ill cancer patient is avoided with the posterolateral approach (14). Despite these advantages, several authors have reported significantly high operative morbidities and mortalities for unknown reasons (9-11,13-17).
The surgical decision-making process and the choice of surgical approach in a patient with spinal metastases is not always straightforward but it’s generally advocated for (I) establishment of histological diagnosis, (II) management of intractable pain, (III) progressive neurological deficits, (IV) chemo and radio-resistant tumors and (V) correction of spinal instability (25-27). Spinal metastases often involve the vertebral body with ventral epidural compression and thus the decision to perform anterior column stabilization (i.e., vertebrectomy with cage reconstruction) in lieu of shorter life expectancy and patient co-morbidities is often contemplated. The determination of spinal instability in metastatic disease also remains a challenge and further complicates the surgical decision-making process. Siegal et al. proposed five criteria indicating spinal instability in metastatic disease based on columns of involvement and presence of iatrogenic instability from prior laminectomy or vertebrectomy (28).This criterion, however, does not include other elements involved in defining spinal instability such as the location of tumor at a transition level and overall radiographic spinal alignment. In an attempt to establish a comprehensive and standardized classification system, Fisher et al. undertook an evidence-based approach and expert consensus from the spine oncology study group to create a spine instability neoplastic score based on patient symptoms and radiographic criteria (29). Future studies utilizing this scoring system will elucidate its validity and applicability.
The difficulty in defining spinal instability in spinal metastatic disease and deciding when to perform anterior column reconstruction is evident in this study in TP group. All patients in this group had three-column involvement with some patients having >50% vertebral body height loss, >50% vertebral body involvement and transitional level location of tumors. As this is a retrospective study, the surgical decision-making thought process was not always available in the records. Nevertheless, this patient group provides a unique opportunity to study the operative and functional outcomes in patients with spinal metastases who do not undergo anterior column reconstruction. Some of the drawbacks of not performing anterior column reconstruction include worsening of spinal instability, tumor recurrence and failure of posterior instrumentation. While these are valid concerns, some may argue that in this patient population with shorter life expectancies, performing a circumferential decompression with or without posterior stabilization may be adequate (20). Weller et al. (20) reported improved neurological outcomes in eight patients with spinal metastasis who underwent a unilateral posterolateral decompression without stabilization. They suggested this limited option for debilitated patients with life expectancies of less than 6 months. This notion is supported in this study by the observation that no patient underwent revision surgery for instrumentation failure, recurrent tumors or worsening of symptoms. Moreover, the ASIA scores, ambulatory status and overall survival were not significantly different between the two groups.
Whether or not performing vertebrectomies with cage reconstruction performed through the posterolateral approaches are associated with more operative and perioperative complications when compared with posterolateral decompression alone is also not well established. It is intuitive to assume that the addition anterior column reconstruction may be associated with increased operative and perioperative complications for a number of reasons including but not limited to (I) increasing length of case, (II) more blood loss, (III) potential risks to surrounding neurovascular structures and (IV) instrumentation failure. In this study the rate of complications of was comparable in both groups and to that of other reported studies (9-11,13-17). As expected, the average blood loss in patients undergoing a vertebrectomy was more than the group who had decompression alone although this difference was not statistically significant.
Moreover, the average length of hospital stay was similar in both groups. Although the number of patients in this study are too small to draw any impactful conclusions, it is noteworthy that performing single or multilevel vertebrectomies does not appear confer increased operative or perioperative complications. Thus, for patients for whom vertebrectomies are appropriate, one should not limit the operative intervention to decompression alone. In the same token, our data suggests that in this patient population with relatively shorter life expectancies, removing the compressive tumors and performing posterior stabilization alone may be adequate even in patients with three-column involvement with radiographic cues for spinal instability. This reinforces the need for well conducted studies designed to establish surgical guidelines in patients with spinal metastases.
There are several limitations in this study. First, as in any retrospective review, recall bias must be considered. Secondly, the number of patients is relatively small to generate statistical power. The heterogeneity in tumor histology also makes it difficult to draw solid conclusions as overall outcomes may have been impacted by the type of tumor.
Conclusions
The posterolateral approach for single or multilevel vertebrectomy with reconstruction does not appear to be associated with more operative or perioperative complications when compared with posterolateral approaches for decompression without anterior column reconstruction. When appropriate, anterior column reconstruction should not be aborted in fear of increasing operative or perioperative complications.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Approval was obtained from the Duke University Institutional Review Board prior to study (No. Pro00066331).
References
- Sundaresan N, DiGiacinto GV, Hughes JE. Surgical approaches to primary and metastatic tumors of spine. In: Schmidek HH, Sweet WH. editors. Operative Neurosurgical Techniques: Indications, Methods, and Results. Orlando, FL: Grune & Stratton, 1988:1525-37.
- Cooper PR, Errico TJ, Martin R, et al. A systematic approach to spinal reconstruction after anterior decompression for neoplastic disease of the thoracic and lumbar spine. Neurosurgery 1993;32:1-8. [Crossref] [PubMed]
- Delamarter RB, Sachs BL, Thompson GH, et al. Primary neoplasms of the thoracic and lumbar spine. An analysis of 29 consecutive cases. Clin Orthop Relat Res 1990.87-100. [PubMed]
- Harrington KD. Anterior cord decompression and spinal stabilization for patients with metastatic lesions of the spine. J Neurosurg 1984;61:107-17. [Crossref] [PubMed]
- Harrington KD. The use of methylmethacrylate for vertebral-body replacement and anterior stabilization of pathological fracture-dislocations of the spine due to metastatic malignant disease. J Bone Joint Surg Am 1981;63:36-46. [Crossref] [PubMed]
- Siegal T, Siegal T. Current considerations in the management of neoplastic spinal cord compression. Spine 1989;14:223-8. [Crossref] [PubMed]
- Siegal T, Siegal T, Robin G, et al. Anterior decompression of the spine for metastatic epidural cord compression: a promising avenue of therapy? Ann Neurol 1982;11:28-34. [Crossref] [PubMed]
- Sundaresan N, Galicich JH, Bains MS, et al. Vertebral body resection in the treatment of cancer involving the spine. Cancer 1984;53:1393-6. [Crossref] [PubMed]
- Cybulski GR, Stone JL, Opesanmi O. Spinal cord decompression via a modified costotransversectomy approach combined with posterior instrumentation for management of metastatic neoplasms of the thoracic spine. Surg Neurol 1991;35:280-5. [Crossref] [PubMed]
- Cybulski GR, Von Roenn KA, D'Angelo CM, et al. Luque rod stabilization for metastatic disease of the spine. Surg Neurol 1987;28:277-83. [Crossref] [PubMed]
- Faccioli F, Lima J, Bricolo A. One-stage decompression and stabilization in the treatment of spinal tumors. J Neurosurg Sci 1985;29:199-205. [PubMed]
- Livingston KE, Perrin RG. The neurosurgical management of spinal metastases causing cord and cauda equina compression. J Neurosurg 1978;49:839-43. [Crossref] [PubMed]
- Perrin RG, McBroom RJ. Anterior versus posterior decompression for symptomatic spinal metastasis. Can J Neurol Sci 1987;14:75-80. [Crossref] [PubMed]
- Shaw B, Mansfield FL, Borges L. One-stage posterolateral decompression and stabilization for primary and metastatic vertebral tumors in the thoracic and lumbar spine. J Neurosurg 1989;70:405-10. [Crossref] [PubMed]
- Jelsma RK, Kirsch PT. The treatment of malignancy of a vertebral body. Surg Neurol 1980;13:189-95. [PubMed]
- Magerl F, Coscia MF. Total posterior vertebrectomy of the thoracic or lumbar spine. Clin Orthop Relat Res 1988.62-9. [PubMed]
- Wiggins GC, Mirza S, Bellabarba C, et al. Perioperative complications with costotransversectomy and anterior approaches to thoracic and thoracolumbar tumors. Neurosurg Focus 2001;11:e4. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Garrido E. Modified costotransversectomy: a surgical approach to ventrally placed lesions in the thoracic spinal canal. Surg Neurol 1980;13:109-13. [PubMed]
- Weller SJ, Rossitch E Jr. Unilateral posterolateral decompression without stabilization for neurological palliation of symptomatic spinal metastasis in debilitated patients. J Neurosurg 1995;82:739-44. [Crossref] [PubMed]
- Akeyson EW, McCutcheon IE. Single-stage posterior vertebrectomy and replacement combined with posterior instrumentation for spinal metastasis. J Neurosurg 1996;85:211-20. [Crossref] [PubMed]
- Ménard V. Causes de la paraplégie dans le mal de Pott, son traitement chirurgical par l'ouverture directe du foyer tuberculeux des vertebres. Rev Orthop 1894;5:47-64.
- Dohn DF. Thoracic spinal cord decompression: alternative surgical approaches and basis of choice. Clin Neurosurg 1980;27:611-23. [Crossref] [PubMed]
- Overby MC, Rothman AS. Anterolateral decompression for metastatic epidural spinal cord tumors. Results of a modified costotransversectomy approach. J Neurosurg 1985;62:344-8. [Crossref] [PubMed]
- Sundaresan N, Rothman A, Manhart K, et al. Surgery for solitary metastases of the spine: rationale and results of treatment. Spine 2002;27:1802-6. [Crossref] [PubMed]
- Sundaresan N, Steinberger AA, Moore F, et al. Indications and results of combined anterior-posterior approaches for spine tumor surgery. J Neurosurg 1996;85:438-46. [Crossref] [PubMed]
- Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine 2001;26:298-306. [Crossref] [PubMed]
- Siegal T, Sietal T. Surgical management of malignant epidural tumors compressing the spinal cord. In: Schmidek HH, Sweet WH. editors. Operative Neurosurgical Techniques: Indications, Methods, and Results. Philadelphia: WB Saunders, 1995:1997-2025.
- Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine 2010;35:E1221-9. [Crossref] [PubMed]


