Inpatient morbidity after spinal deformity surgery in patients with movement disorders
Introduction
Movement disorders (MD) are a group of neurologic syndromes characterized by excess or slowness of movements. The most common of these disorders is Parkinson’s disease (PD), which is the second most common neurodegenerative disorder after Alzheimer’s (1). Currently, it is estimated that approximately 1% of the population above 60 has PD, and prevalence increases to almost 4% by the age of 80 (2). Other less common MD include tremors, restless leg syndrome, primary and secondary dystonia, chorea, tics, and others (3). Patients with MD may develop spinal deformities during the course of the disease, which may be the result of both neuromuscular and degenerative conditions (4,5). Some of these deformities include cervical positive sagittal malalignment (6), sagittal spinopelvic malalignment (5), and global coronal malalignment (7). PD patients are known to suffer from spinal deformities such as kyphoscoliosis, camptocormia (forward flexion of the spine when standing or walking only), cervical dystonia, and Pisa syndrome (lateral flexion of the trunk when sitting or standing) (4). Treatment of these spinal deformities is limited and poorly responsive to medications including dopaminergics.
Surgery for spinal deformity has shown superiority to non-operative treatment in various investigations (8-11). Although patients with MD have been shown to have a higher incidence of cervical, sagittal, and coronal malalignment than patients without this condition of the same age (5-7), there is a paucity of literature on positive and negative outcomes regarding spinal deformity surgery in patients with MD (4,12). Thus, the purpose of this investigation is to report inpatient perioperative complication rates of patients with MD undergoing spinal deformity surgery.
Methods
Study design
This is an observational retrospective cohort study comparing inpatient morbidity after spinal deformity surgery in patients with and without MD. The study received local institutional review board approval (No. IRB00096323).
Data source
The Nationwide Inpatient Sample (NIS) databases from 2002 to 2011 were used to identify patients over the age of 21 who underwent primary or revision spinal deformity surgery. The NIS is an inpatient healthcare database from the United States, containing discharge information from over 7 million hospital stays each year. This information is provided by a 20% sample of nonfederal hospitals in the country, and multiple studies have used it to examine short-term outcome in spine surgery (13-20). Diagnostic and procedural information is available in the form of International Classification of Diseases 9th Version (ICD-9) codes.
Inclusion and exclusion criteria
Patients who underwent spinal fusion (cervical, thoracic, or lumbosacral) for spinal deformity were identified via use of the following codes: acquired postural kyphosis (737.10), postlaminectomy kyphosis (737.12), kyphosis not elsewhere classified (737.19), acquired postural lordosis (737.20), postlaminectomy lordosis (737.21), other postsurgical lordosis (737.22), idiopathic scoliosis and kyphoscoliosis (737.30), progressive infantile idiopathic scoliosis (737.32), thoracogenic scoliosis (737.34), scoliosis not elsewhere classified (737.39), unspecified curvature of spine (737.40), kyphosis from secondary cause (737.41), lordosis from secondary cause (737.42), and neuromuscular scoliosis (737.43). Patients younger than 21 years of age and non-elective admissions were excluded.
Recorded data and outcome measures
Two groups were established in this study: no MD (control group) and MD group (cases). MD diagnoses included PD (332.0), secondary parkinsonism (332.1), essential and other specified forms of tremor (333.1), myoclonus (333.2), tics of organic origin (333.3), Huntington’s chorea (333.4), other choreas (333.5), genetic torsion dystonia (333.7), athetoid cerebral palsy (333.71), unspecified extrapyramidal disease and abnormal MD (333.90), Stiff-man syndrome (333.91), Restless leg syndrome (333.94), and other extrapyramidal diseases and abnormal MD (333.99).
Individual patient information such as age, sex, race, insurance status and Charlson Comorbidity Index (CCI) score was gathered. Operative parameters included performing a “complex procedure” (defined as revision surgery, osteotomy, and/or fusion of 8 or more spinal segments), use of packed red blood cell transfusion, and use of recombinant human bone morphogenetic protein-2 (rh-BMP-2).
The primary endpoint of this study was the development of at least one in-hospital perioperative complication. These included implant-related complications, reintubation, delirium, deep vein thrombosis, pulmonary embolism, pneumonia, myocardial infarction, acute kidney injury, surgical site infection, sepsis, stroke, adult respiratory distress syndrome, and acute post-hemorrhagic anemia.
Statistical analysis
Variables were compared between groups via use of unpaired Student’s t-tests, chi-squared tests, or Fisher’s exact tests as appropriate. A multiple logistic regression model was used to assess the effect of MD on outcome. Results of regression analysis are presented as odds ratios (OR) with 95% confidence intervals (CI). All analyses were done in Stata SE 12 (StataCorp LP, College Station, Texas), and the alpha level was set at 0.05.
Results
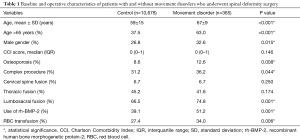
A total of 11,043 patients met inclusion criteria for this study, out of whom 365 patients (3.3%) had MD (Table 1). These disorders included PD (1.5%), restless leg syndrome (1.4%), essential and other specified forms of tremor (0.2%), and others (0.2%). Patients with MD were on average 8 years older than the control group (67 vs. 59 years, P<0.001) and more likely to be male (32.6% vs. 26.8%, P=0.015). The median CCI score for both groups was 0 and there were no statistically significant differences (P=0.146). Osteoporosis was present in 12.6% of patients with MD and 8.6% of patients in the control group (P=0.008).
Full table
Complex procedures, defined as revision surgery, osteotomy, or instrumentation of 8 or more spinal segments, were performed in 31.4% of all patients (36.2% of patients with MD and 31.2% of controls, P=0.044). The majority of patients had fusion of their lumbosacral spine (74.8% of patients in the MD group and 66.5% of patients in the control group, P=0.001), followed by the thoracic spine in 45.2% and 41.6% of patients, respectively (P=0.174); the cervical spine was fused in only 6.7% of cases (P=0.250). The rate of rh-BMP-2 use (P=0.001) and packed red blood cell transfusion use (P=0.006) was also significantly different between cohorts, being more frequent in the MD group.
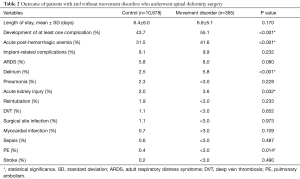
Average length of stay was 6.8 days for patients with MD and not significantly different from 6.4 days for controls (P=0.170) (Table 2). On the other hand, 55.1% of MD patients developed at least one perioperative complication, versus 43.7% in the control group (P<0.001). The most common complication was acute post-hemorrhagic anemia, which occurred in 31.9% of all patients (41.6% in MD and 31.5% in the control group, P<0.001). Other complications that were more common in patients with MD included delirium (P<0.001), acute kidney injury (P=0.032), and pulmonary embolism (P=0.014).
Full table
After controlling for patient age, sex, osteoporosis, complex procedures, fusion to the lumbosacral spine, use of rh-BMP-2, and use of RBC transfusion, patients with MD were 1.3 times more likely to develop a complication compared to patients without MD (OR, 1.27; 95% CI, 1.02–1.59; P=0.032) on multiple logistic regression modeling.
Subanalysis for PD
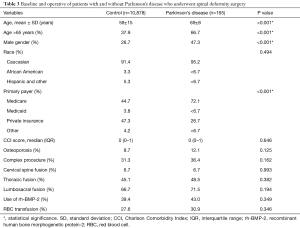
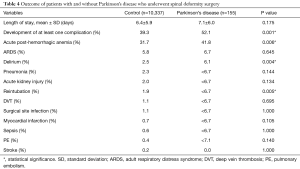
Out of the 365 patients with MD, 165 had PD (45.2% of all cases of MD or 1.5% of the entire sample). Patients with PD were on average 10 years older than patients without PD (69 vs. 59 years of age, P<0.001) (Table 3). Preoperative comorbidities and operative parameters (location, number of levels fused, use of osteotomy, revision status, and others) were not different between cohorts. The complication rate was 52.1% for patients with PD and 39.3% for patients without PD (P=0.001) (Table 4). The most common complication was acute post-hemorrhagic anemia, which occurred in 31.9% of all patients (41.8% in PD patients and 31.7% in the control group, P=0.006). Other complications that were more common in patients with PD included delirium (P=0.004) and reintubation (P=0.005). After controlling for patient age, sex, and primary payer, patients with PD were 1.6 times more likely to develop a complication compared to patients without PD (OR, 1.55; 95% CI, 1.14–2.28; P=0.005).
Full table
Full table
Discussion
MD are considered an important cause of chronic neurological disability (3). Wenning et al. found an overall prevalence of MD of 28.0% (27.6% in men and 28.3% in women) in patients aged 50 to 89 years (3). Specifically, the prevalence of tremor was 14.5% (most commonly exaggerated physiological postural tremor), followed by restless leg syndrome in 10.8% of patients, parkinsonism in 7.0%, primary and secondary dystonia in 1.8%, and chorea and tics in less than 1.0% each (3). Patients with MD, particularly PD, are known to suffer from spinal deformities such as kyphoscoliosis, camptocormia (forward flexion of the spine when standing or walking only), antecollis (cervical dystonia), and Pisa syndrome (lateral flexion of the trunk when sitting or standing) (4). Although there is limited literature on outcomes after spinal surgery in patients with MD, studies have suggested a relatively high revision rate (due to mechanical complications or pseudoarthrosis) (21) and high perioperative complication rate (22). In this study, we used a large inpatient database to examine perioperative complication rates after spinal deformity surgery in patients with and without MD, finding that the former had statistically significant higher complication rate.
The rates of acute post-hemorrhagic anemia (41.6% vs. 31.5%), delirium (5.8% vs. 2.5%), acute kidney injury (3.6% vs. 2.0%), and pulmonary embolism (<3.0% vs. 0.4%) were significantly higher in patients with MD compared to the control group. One of the possible explanations for excessive bleeding in patients with MD and development of acute post-hemorrhagic anemia is the need of multi-level instrumentation (oftentimes from the thoracic spine to the ilium) (4,22). Koller et al. reported outcomes of 23 patients with PD who underwent surgery for deformity. Eighteen patients had multilevel surgery, with a mean of 6.1± instrumented levels (range, 1–16); 19 patients from the total cohort (82.6%) had fusion to the sacrum. In a similar investigation, Bourghli et al. examined 12 patients (6 primary surgeries and 6 revision) with PD who underwent deformity surgery (23). All patients underwent posterior segmental instrumentation from T2 to the sacrum (16 levels) (23). In our study, complex procedures (involving revision surgery, osteotomy, and/or fusion of 8 or more spinal segments) were significantly more common in patients with MD (36.2% vs. 31.2%, P=0.044), as well as the need for red blood cell transfusion (34.0% vs. 27.4%, P=0.006). Additionally, a higher proportion of patients underwent fusion of the lumbosacral spine when compared to controls (74.8% vs. 66.5%, P=0.001).
Delirium is defined as “an acute disturbance in attention and awareness that fluctuates and is accompanied by an additional disturbance in cognition” (24). Postoperative delirium was reported in 3/23 patients (13.0%) in the study of Koller et al. (23 patients with PD who underwent spinal deformity) (22) . Similar to our findings of greater risk of delirium in perioperative patients with MD, Lubomski et al. retrospectively compared 5,637 admissions of patients with the MD to 8,143 controls and found that patients with PD were 5 times more likely to be treated for delirium than the control group (25). In another investigation, Gerlach et al. prospectively studied all patients with PD who were admitted to a Dutch teaching hospital during a 1-year period (26). Of the 46 patients that were included in their study, 48% developed a complication, with the most common being confusion/delirium in 24% of all patients and infection in 15% (26). Delirium is a common manifestation in perioperative PD patients, which can be associated to medication errors or infections suffered during hospitalization in addition to the associated cognitive impairment in PD patients (27).
Acute kidney injury and pulmonary embolism were also more common in patients with MD than in controls. Naik et al. estimated the risk of kidney failure to be 3.9% after spine surgery, and they identified hypertension as the only preoperative risk factor for this occurrence (28). Although the exact mechanism through which postoperative kidney failure was reported more frequently in patients with MD is unknown, future research into the matter is definitively encouraged. On the other hand, increased risk of embolism may be explained by the fact that MD often result in rigidity, bradykinesia, and immobility (29). Additionally, patients with MD underwent more “complex” procedures than controls in the present study, which may have also accounted for the increased risk of pulmonary embolism.
Overall complications after spinal surgery for MD range from 33–86% (21,22). In the present study, the overall complication rate was 55.1% in patients with MD, and these disorders were found to be independent predictors of adverse events. However, despite the increased complication rate, this not appear to increase length of stay. Although the increased prevalence of severe deformity and need for longer constructs in patients with MD may contribute to the increased risk of complications, alterations in cognition and drugs interactions, may also play a role, and future research into preoperative optimization of this population and complication avoidance is encouraged.
Limitations
There are several limitations to the present study. Although the NIS has been used on various studies in the spine surgery literature, it has been criticized for lack of “granularity” or more specific parameters such as curve types, data on preoperative symptoms, radiographic parameters, and others. Additionally, no long-term follow-up is available, as all information is inpatient data only. Nonetheless, the large patient sample included within the NIS allows for identification and analysis of more “uncommon” events such as spinal deformity surgery in patients with MD.
Conclusions
Patients with MD may have a significantly higher risk of immediate perioperative complications after spinal deformity surgery. Acute post-hemorrhagic anemia, delirium, acute kidney injury, and pulmonary embolism may be more frequent in patients with MD, and future research into preoperative optimization and complication avoidance is encouraged. However, the higher risk of complications did not seem to affect length of hospital stay.
Acknowledgements
None.
Footnote
Conflicts of Interest: CR Goodwin is a UNCF Merck Postdoctoral fellow and has received an award from the Burroughs Wellcome Fund and the Johns Hopkins Neurosurgery Pain Research Institute. DM Sciubba has consulting relationships with Medtronic, Globus, DePuy, Stryker and Orthofix. The other authors have no conflicts of interest to declare.
Ethical Statement: The study received local institutional review board approval (No. IRB00096323).
References
- Hattori N. Movement disorders: advances in 2015. Lancet Neurol 2016;15:8-9. [Crossref] [PubMed]
- de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol 2006;5:525-35. [Crossref] [PubMed]
- Wenning GK, Kiechl S, Seppi K, et al. Prevalence of movement disorders in men and women aged 50-89 years (Bruneck Study cohort): a population-based study. Lancet Neurol 2005;4:815-20. [Crossref] [PubMed]
- Ha Y, Oh JK, Smith JS, et al. Impact of Movement Disorders on Management of Spinal Deformity in the Elderly. Neurosurgery 2015;77 Suppl 4:S173-85. [Crossref] [PubMed]
- Oh JK, Smith JS, Shaffrey CI, et al. Sagittal spinopelvic malalignment in Parkinson disease: prevalence and associations with disease severity. Spine (Phila Pa 1976) 2014;39:E833-41. [Crossref] [PubMed]
- Moon BJ, Smith JS, Ames CP, et al. Prevalence and type of cervical deformities among adults with Parkinson's disease: a cross-sectional study. J Neurosurg Spine 2016;24:527-34. [Crossref] [PubMed]
- Choi HJ, Smith JS, Shaffrey CI, et al. Coronal plane spinal malalignment and Parkinson's disease: prevalence and associations with disease severity. Spine J 2015;15:115-21. [Crossref] [PubMed]
- Acaroglu E, Yavuz AC, Guler UO, et al. A decision analysis to identify the ideal treatment for adult spinal deformity: is surgery better than non-surgical treatment in improving health-related quality of life and decreasing the disease burden? Eur Spine J 2016;25:2390-400. [Crossref] [PubMed]
- Smith JS, Lafage V, Shaffrey CI, et al. Outcomes of Operative and Nonoperative Treatment for Adult Spinal Deformity: A Prospective, Multicenter, Propensity-Matched Cohort Assessment With Minimum 2-Year Follow-up. Neurosurgery 2016;78:851-61. [Crossref] [PubMed]
- Sciubba DM, Scheer JK, Yurter A, et al. Patients with spinal deformity over the age of 75: a retrospective analysis of operative versus non-operative management. Eur Spine J 2016;25:2433-41. [Crossref] [PubMed]
- Scheer JK, Smith JS, Clark AJ, et al. Comprehensive study of back and leg pain improvements after adult spinal deformity surgery: analysis of 421 patients with 2-year follow-up and of the impact of the surgery on treatment satisfaction. J Neurosurg Spine 2015;22:540-53. [Crossref] [PubMed]
- Sarkiss CA, Fogg GA, Skovrlj B, et al. To operate or not?: A literature review of surgical outcomes in 95 patients with Parkinson's disease undergoing spine surgery. Clin Neurol Neurosurg 2015;134:122-5. [Crossref] [PubMed]
- De la Garza-Ramos R, Jain A, Kebaish KM, et al. Inpatient morbidity and mortality after adult spinal deformity surgery in teaching versus nonteaching hospitals. J Neurosurg Spine 2016;25:15-20. [Crossref] [PubMed]
- De la Garza-Ramos R, Passias PG, Schwab FJ, et al. The effect of July admission on inpatient morbidity and mortality after adult spinal deformity surgery. Int J Spine Surg 2016;10:3. [Crossref] [PubMed]
- De la Garza-Ramos R, Samdani AF, Sponseller PD, et al. Visual loss after corrective surgery for pediatric scoliosis: incidence and risk factors from a nationwide database. Spine J 2016;16:516-22. [Crossref] [PubMed]
- De la Garza-Ramos R, Abt NB, Kerezoudis P, et al. Provider volume and short-term outcomes following surgery for spinal metastases. J Clin Neurosci 2016;24:43-6. [Crossref] [PubMed]
- Nandyala SV, Marquez-Lara A, Fineberg SJ, et al. Complications after lumbar spine surgery between teaching and nonteaching hospitals. Spine (Phila Pa 1976) 2014;39:417-23. [Crossref] [PubMed]
- Marquez-Lara A, Nandyala SV, Hassanzadeh H, et al. Sentinel events in cervical spine surgery. Spine (Phila Pa 1976) 2014;39:715-20. [Crossref] [PubMed]
- Marquez-Lara A, Nandyala SV, Hassanzadeh H, et al. Sentinel Events in Lumbar Spine Surgery. Spine (Phila Pa 1976) 2014. [Epub ahead of print]. [PubMed]
- Nandyala SV, Marquez-Lara A, Fineberg SJ, et al. Perioperative characteristics and outcomes of patients undergoing anterior cervical fusion in July: analysis of the "July effect". Spine (Phila Pa 1976) 2014;39:612-7. [Crossref] [PubMed]
- Babat LB, McLain RF, Bingaman W, et al. Spinal surgery in patients with Parkinson's disease: construct failure and progressive deformity. Spine (Phila Pa 1976) 2004;29:2006-12. [Crossref] [PubMed]
- Koller H, Acosta F, Zenner J, et al. Spinal surgery in patients with Parkinson's disease: experiences with the challenges posed by sagittal imbalance and the Parkinson's spine. Eur Spine J 2010;19:1785-94. [Crossref] [PubMed]
- Bourghli A, Guerin P, Vital JM, et al. Posterior spinal fusion from T2 to the sacrum for the management of major deformities in patients with Parkinson disease: a retrospective review with analysis of complications. J Spinal Disord Tech 2012;25:E53-60. [Crossref] [PubMed]
- Vardy ER, Teodorczuk A, Yarnall AJ. Review of delirium in patients with Parkinson's disease. J Neurol 2015;262:2401-10. [Crossref] [PubMed]
- Lubomski M, Rushworth RL, Tisch S. Hospitalisation and comorbidities in Parkinson's disease: a large Australian retrospective study. J Neurol Neurosurg Psychiatry 2015;86:324-30. [Crossref] [PubMed]
- Gerlach OH, Broen MP, Weber WE. Motor outcomes during hospitalization in Parkinson's disease patients: a prospective study. Parkinsonism Relat Disord 2013;19:737-41. [Crossref] [PubMed]
- Fernandez HH, Trieschmann ME, Friedman JH. Treatment of psychosis in Parkinson's disease: safety considerations. Drug Saf 2003;26:643-59. [Crossref] [PubMed]
- Naik BI, Colquhoun DA, McKinney WE, et al. Incidence and risk factors for acute kidney injury after spine surgery using the RIFLE classification. J Neurosurg Spine 2014;20:505-11. [Crossref] [PubMed]
- Mosewich RK, Rajput AH, Shuaib A, et al. Pulmonary embolism: an under-recognized yet frequent cause of death in parkinsonism. Mov Disord 1994;9:350-2. [Crossref] [PubMed]


