The genetic implication of scoliosis in osteogenesis imperfecta: a review
Introduction
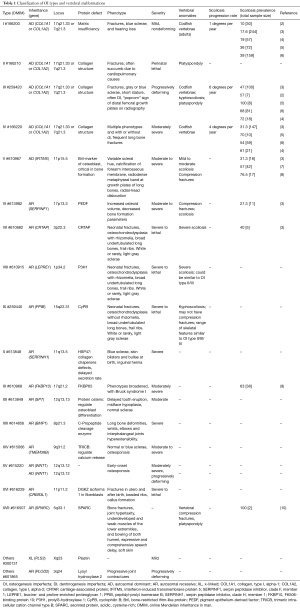
Osteogenesis imperfecta (OI) is a kind of heritable skeletal dysplasia, which is often called “fragile bone”. It affects about 1 in 5,000 to 20,000 births (1), and most cases are caused by mutation of collagen related genes, non-collagen genes account for less than 10% of OI patients (Table 1). The classical phenotypes of OI include frequent long bone fractures, vertebral compression fractures, short stature, blue sclera and dentinogenesis imperfecta (DI) (11). Patients can also have other manifestations, such as scoliosis, unilateral spinal anesthesia (12), among which scoliosis is commonly seen.
Full table
According to previous investigations, the prevalence of scoliosis in OI varies from 26% to 74.5% (2,3,5-7,11,13,14). The severity and prevalence of scoliosis in different types of OI is various (Table 1), and the type III patients often had higher prevalence of severe scoliosis than type I and IV (2,3,6).
The outset years of scoliosis in OI cases ranged from 2 to 65 years (15), always the spinal malformation progresses rapidly after 5 years old or after the spinal curve exceeds 50 degrees (16). Although scoliosis was rare before 6 years of age (17), some types of OI can also have scoliosis just after born (18).
The curvature of scoliosis in OI was different varying from 7 to 105 degrees (19). According to a national cross-sectional study by Karbowski (14), 73.7% was mainly mild (<40 degrees), while 10.5% showed moderate (<60 degrees), 9.2% severe (<80 degrees) and 6.6% very severe deformity (>80 degrees). The vertebral deformities included codfish or wedge-shaped vertebrae (20) which were mostly common, and platyspondylia. Another study indicated that there were four types of vertebral body deformities including biconcave, flattened, wedged and unclassifiable vertebrae. The number of biconcave vertebrae (normally six or more) may indicate the severity and possibility of scoliosis (21).
Although scoliosis develops indolently, once the malformations evolve, they tend to be progressive and have numerous influence on the patients’ life, such as pulmonary function and height (22). The treatment is ineffective in severely affected individuals who have minimal cortical bone (23), so it is necessary to prevent spinal curvature progression before severe complications arise (16,17). We are going to explore the tendency and severity of scoliosis, and give some interventions before scoliosis progressing in different types of OI (24). This review will be the first to give an integrated genetic landscape and aim to provide a basic knowledge of scoliosis in OI (25).
Genetic variants and pathogenesis
There are 19 types of OI according to genetic variants, the pathogenesis is not fully understood yet as shown in Figure 1. Based on the mechanism, OI can be divided into five groups (26). According to previous research, all of the groups and 16 types of the 19 types were reported to be manifest with scoliosis.
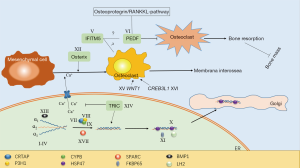
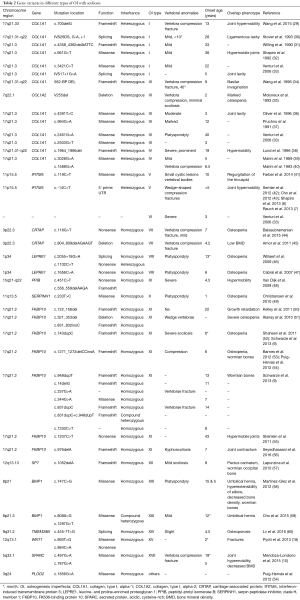
In the first group, OI is mainly caused by defects in collagen synthesis, structure, or processing including type I–IV and XIII. Most of OI patients have mutations in type I collagen related genes. Based on severity, OI is classified into four types (27). As shown in Table 1, patients with OI type I to IV always have variants in either collagen, type I, alpha-1 (COL1A1) or collagen, type I, alpha-2 (COL1A2). The production of type 1 collagen α1 or α2 chains would decrease. Patients with type I OI always have lower bone mineral density (BMD), thinner cortexes and reduced trabecular number (28) which would cause vertebra compression fracture. Together with joint hypermobility, patients manifested with scoliosis as shown in Table 2. Type II OI is also caused by mutations in COL1A1 or COL1A2, but this type is always too lethal to observe bone change and scoliosis. Type III has severely deforming and higher prevalence of scoliosis with vertebra compression and platyspondyly. Bisphosphonate treatment could decrease Cobb angle progression rates in type III at early age (24). Type IV can also have vertebra compression and severe scoliosis. OI type XIII is mainly caused by BMP1 defects which leads to retention of the C-propeptide (61). Scoliosis with umbilical hernia and platyspondyly were reported at early age (58).
Full table
In the second group, OI is mainly caused by defects in collagen modification including type VII–IX, XIV and XVII. The collagen prolyl 3-hydroxylation complex which consisted of three proteins in a 1:1:1 ratio of prolyl3-hydroxylase 1 (P3H1), cartilage-associated protein (CRTAP), and cyclophilin B (CyPB) has a significant collagen post-translational over-modification role (62). Each of those protein is encoded by CRTAP, LEPRE1 and PPIB. Defects of these three genes which cause delay of collagen helix folding could lead to OI type VII, VIII and IX (63). Defects of secreted protein, acidic, cysteine-rich (SPARC) which encoded by SPARC also could lead to delay of collagen folding, this type OI is considered to be type XVII (10). Type XIV is caused by TMEM38B mutations. The mechanism has not been completely elucidated. According to recent studies, TMEM38B mutations could inhibit calcium release, abnormal calcium signaling would decrease osteoblast growth and differentiation (64). Meanwhile post-translational modification of collagen would be influenced by calcium alteration of endoplasmic reticulum (26). In those types, patients with scoliosis always have low BMD as shown in Table 2.
In the third group, OI is mainly caused by defects in collagen folding and cross-linking including type X, XI and type caused by PLOD2 mutation. OI type X is mainly caused by mutation of SERPINH1 which encodes HSP47. HSP47 is important in stabilizing folded collagen and transferring to Golgi (49). This type of OI could lead to platyspondyly and scoliosis at early age. Like SERPINH1, FKBP10 is another important gene in procollagen modification (9). Its deficiency could lead to OI type XI. Associated with FKBP10, PLOD2 which encodes LH2 is another gene which could cause OI (54). Scoliosis is also very common in both types.
In the fourth group, OI is mainly caused by defects in bone mineralisation including type V and VI. Mutations of interferon-induced transmembrane protein 5 (IFITM5) could cause autosomal-dominant OI V. IFITM5 has close relationship with osteoblast, which may elucidate hyperplastic callus formation and membrana interossea ossification of forearms after injury (65). Patients with scoliosis could have cystic lesions vertebral bodies or vedge-shaped compression fractures (41). Connected with IFITM5, SERPINF1 which underlying OI type VI encodes protein pigment epithelium-derived factor (PEDF) (41). PEDF plays an important role in osteoprotegerin/RANKLE-pathway (66). Some studies had shown that decreased PEDF level may lead to activated osteoclast increased and thus induced bone resorption (67,68). This type OI could have severe scoliosis (33).
In the fifth group, OI is mainly caused by defects in osteoblast development with collagen insufficiency including type XII, XV and XVI. SP7 which encodes protein Osterix is target gene of Wnt pathway. Scoliosis in OI type XII with SP7 mutation was also reported (57), osteoblast development defects were considered to happen in this progress. Both heterozygous and homozygous WNT1 mutations could lead to OI type XV. As a member of Wnt family, mutations of WNT1 could cause complex signaling pathway defects in bone formation. In this type, scoliosis with early onset osteoporosis was reported (18). Just like WNT1, CREB3L1 mutation could also influence osteoblast development which may cause OI type XVI (69). But no scoliosis was reported yet. As OI type XVI, PLS3 mutation could lead to OI manifesting with osteoporosis and fractures (70). The exact mechanism is not known and report with scoliosis was not found yet.
Mechanism of scoliosis
The mechanism of scoliosis in OI has not been clarified, it is thought that there are some triggering factors such as vertebral microfractures caused by vertebral growth plates injuries or bone fragility. Some other factors like length inequality, pelvic obliquity, ligamentous laxity and inter-vertebral disc abnormalities would lead to scoliotic progression.
The vertebral body malformation may cause abnormal spinal curve in OI. Wedged vertebrae had been reported in OI patients representing kyphosis and quadriparesis (71). Fragile bone and fracture could lead to deformities in some severe OI forms, for example scoliosis (72). Although this is very common in OI, scoliosis patients can have no spinal fracture (32,59).
Osteopenia is also very common in OI patients which might be the pathology of scoliosis because of vertebral fragility (73). Some studies have shown the positive correlation of scoliosis with Z-score BMD and BMI (74). In Col1a1Jrt/+ mice model with OI and Ehlers-Danlos Syndrome (EDS) (75), the scoliosis mice had lower BMD and bone mineral content (BMC) compared with age-matched +/+ littermates which may lead to the early and rapid progressive malformation of vertebrae body.
There were many other factors which may influence scoliosis in OI. According to a retrospective study (11), scoliosis was significantly associated with age, whereas other clinical characteristics such as gender, weight, SDI were not. In some cases (76), scoliosis and vertebral body compression only happened during growth. Engelbert (4) found that the age of first achieving scoliosis was associated with the age of anti-gravity motor milestone, such as “supported sitting”. The connection may be caused by mechanical loads change. Some other studies also shown that the prevalence of scoliosis at maturity was not influenced by bisphosphonate treatment history although the treatment could decrease the progression (24).
Another important reason is increased mechanical strains during childhood. Mechanical loads with osteopenia can cause bone remodeling and progressive deformations, and the pedicle elongation is the most common result. Some OI cases with severe hyperlordosis had been reported to be caused by lumbar pedicle elongation and spondylolisthesis (77). Some other researchers proposed mechanostat model to illustrate bone deformations cause by mechanical forces (78).
Joint hyperlaxity can lead to scoliosis and chest malformations (73). In a subset of OI (79), patients with OI/EDS can have scoliosis because of ligamentous laxity, dislocations of other joints and mild osteopenia, with a few fractures. This may be caused by mutation of exon 6 fromαchain which lead to N-propeptide retention.
Conclusions
Most of the types OI could manifest with scoliosis, with type III patients have higher prevalence and type XV has the earliest scoliosis onset age. The exact mechanism of scoliosis in OI is complex and has not been fully elucidated. Based on current studies, scoliosis is mainly influenced by OI type, osteopenia, age, BMD, BMC, mechanical strains and ligamentous laxity.
Acknowledgements
Funding: This research was funded by National Natural Science Foundation of China (81501852, 81472046, 81772299), Beijing Natural Science Foundation (7172175), Beijing nova program (Z161100004916123), Beijing nova program interdisciplinary collaborative project (xxjc201717), 2016 Milstein Medical Asian American Partnership Foundation Fellowship Award in Translational Medicine, The Central Level Public Interest Program for Scientific Research Institute (2016ZX310177), PUMC Youth Fund & the Fundamental Research Funds for the Central Universities (3332016006), CAMS Initiative Fund for Medical Sciences (2016-I2M-3-003), the Distinguished Youth foundation of Peking Union Medical College Hospital (JQ201506), the 2016 PUMCH Science Fund for Junior Faculty (PUMCH-2016-1.1).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Byers PH, Steiner RD. Osteogenesis imperfecta. Annu Rev Med 1992;43:269-82. [Crossref] [PubMed]
- Arponen H, Mäkitie O, Waltimo-Sirén J. Association between joint hypermobility, scoliosis, and cranial base anomalies in paediatric Osteogenesis imperfecta patients: a retrospective cross-sectional study. BMC Musculoskelet Disord 2014;15:428. [Crossref] [PubMed]
- Patel RM, Nagamani SC, Cuthbertson D, et al. A cross-sectional multicenter study of osteogenesis imperfecta in North America - results from the linked clinical research centers. Clin Genet 2015;87:133-40. [Crossref] [PubMed]
- Engelbert RH, Uiterwaal CS, van der Hulst A, et al. Scoliosis in children with osteogenesis imperfecta: influence of severity of disease and age of reaching motor milestones. Eur Spine J 2003;12:130-4. [PubMed]
- Wekre LL, Kjensli A, Aasand K, et al. Spinal deformities and lung function in adults with osteogenesis imperfecta. Clin Respir J 2014;8:437-43. [Crossref] [PubMed]
- Anissipour AK, Hammerberg KW, Caudill A, et al. Behavior of scoliosis during growth in children with osteogenesis imperfecta. J Bone Joint Surg Am 2014;96:237-43. [Crossref] [PubMed]
- Rauch F, Moffatt P, Cheung M, et al. Osteogenesis imperfecta type V: marked phenotypic variability despite the presence of the IFITM5 c.-14C>T mutation in all patients. J Med Genet 2013;50:21-4. [Crossref] [PubMed]
- Shapiro JR, Lietman C, Grover M, et al. Phenotypic variability of osteogenesis imperfecta type V caused by an IFITM5 mutation. J Bone Miner Res 2013;28:1523-30. [Crossref] [PubMed]
- Schwarze U, Cundy T, Pyott SM, et al. Mutations in FKBP10, which result in Bruck syndrome and recessive forms of osteogenesis imperfecta, inhibit the hydroxylation of telopeptide lysines in bone collagen. Hum Mol Genet 2013;22:1-17. [Crossref] [PubMed]
- Mendoza-Londono R, Fahiminiya S, Majewski J, et al. Recessive osteogenesis imperfecta caused by missense mutations in SPARC. Am J Hum Genet 2015;96:979-85. [Crossref] [PubMed]
- Ben Amor IM, Roughley P, Glorieux FH, et al. Skeletal clinical characteristics of osteogenesis imperfecta caused by haploinsufficiency mutations in COL1A1. J Bone Miner Res 2013;28:2001-7. [Crossref] [PubMed]
- Baranovic S, Lubina IZ, Vlahovic T, et al. Unilateral spinal anaesthesia in a patient with Osteogenesis Imperfecta with a lower leg fracture: a case report. Injury 2013;44 Suppl 3:S49-51. [Crossref] [PubMed]
- Radunovic Z, Wekre LL, Steine K. Right ventricular and pulmonary arterial dimensions in adults with osteogenesis imperfecta. Am J Cardiol 2012;109:1807-13. [Crossref] [PubMed]
- Karbowski A, Schwitalle M, Eckardt A. Scoliosis in patients with osteogenesis imperfecta: a federal nation-wide cross-sectional study. Z Orthop Ihre Grenzgeb 1999;137:219-22. [Crossref] [PubMed]
- Bischoff H, Freitag P, Jundt G, et al. Type I osteogenesis imperfecta: diagnostic difficulties. Clin Rheumatol 1999;18:48-51. [Crossref] [PubMed]
- Norimatsu H, Mayuzumi T, Takahashi H. The development of the spinal deformities in osteogenesis imperfecta. Clin Orthop Relat Res 1982.20-5. [PubMed]
- Benson DR, Newman DC. The spine and surgical treatment in osteogenesis imperfecta. Clin Orthop Relat Res 1981.147-53. [PubMed]
- Pyott SM, Tran TT, Leistritz DF, et al. WNT1 mutations in families affected by moderately severe and progressive recessive osteogenesis imperfecta. Am J Hum Genet 2013;92:590-7. [Crossref] [PubMed]
- Cristofaro RL, Hoek KJ, Bonnett CA, et al. Operative treatment of spine deformity in osteogenesis imperfecta. Clin Orthop Relat Res 1979.40-8. [PubMed]
- McPherson E, Clemens M. Bruck syndrome (osteogenesis imperfecta with congenital joint contractures): review and report on the first North American case. Am J Med Genet 1997;70:28-31. [Crossref] [PubMed]
- Ishikawa S, Kumar SJ, Takahashi HE, et al. Vertebral body shape as a predictor of spinal deformity in osteogenesis imperfecta. J Bone Joint Surg Am 1996;78:212-9. [Crossref] [PubMed]
- Widmann RF, Bitan FD, Laplaza FJ, et al. Spinal deformity, pulmonary compromise, and quality of life in osteogenesis imperfecta. Spine (Phila Pa 1976) 1999;24:1673-8. [Crossref] [PubMed]
- Yong-Hing K, Macewen GD. Scoliosis associated with osteogenesis imperfecta. J Bone Joint Surg Br 1982;64:36-43. [PubMed]
- Sato A, Ouellet J, Muneta T, et al. Scoliosis in osteogenesis imperfecta caused by COL1A1/COL1A2 mutations - genotype-phenotype correlations and effect of bisphosphonate treatment. Bone 2016;86:53-7. [Crossref] [PubMed]
- Phan K, Mobbs RJ. Systematic reviews and meta-analyses in spine surgery, neurosurgery and orthopedics: guidelines for the surgeon scientist. J Spine Surg 2015;1:19-27. [PubMed]
- Forlino A, Marini JC. Osteogenesis imperfecta. Lancet 2016;387:1657-71. [Crossref] [PubMed]
- Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet 1979;16:101-16. [Crossref] [PubMed]
- Hald JD, Folkestad L, Harsløf T, et al. Skeletal phenotypes in adult patients with osteogenesis imperfecta-correlations with COL1A1/COL1A2 genotype and collagen structure. Osteoporos Int 2016;27:3331-41. [Crossref] [PubMed]
- Wang X, Pei Y, Dou J, et al. Identification of a novel COL1A1 frameshift mutation, c.700delG, in a Chinese osteogenesis imperfecta family. Genet Mol Biol 2015;38:1-7. [Crossref] [PubMed]
- Stover ML, Primorac D, Liu SC, et al. Defective splicing of mRNA from one COL1A1 allele of type I collagen in nondeforming (type I) osteogenesis imperfecta. J Clin Invest 1993;92:1994-2002. [Crossref] [PubMed]
- Willing MC, Cohn DH, Byers PH. Frameshift mutation near the 3' end of the COL1A1 gene of type I collagen predicts an elongated Pro alpha 1(I) chain and results in osteogenesis imperfecta type I. J Clin Invest 1990;85:282-90. [Crossref] [PubMed]
- Shapiro JR, Stover ML, Burn VE, et al. An osteopenic nonfracture syndrome with features of mild osteogenesis imperfecta associated with the substitution of a cysteine for glycine at triple helix position 43 in the pro alpha 1(I) chain of type I collagen. J Clin Invest 1992;89:567-73. [Crossref] [PubMed]
- Venturi G, Tedeschi E, Mottes M, et al. Osteogenesis imperfecta: clinical, biochemical and molecular findings. Clin Genet 2006;70:131-9. [Crossref] [PubMed]
- Wang Q, Forlino A, Marini J C. Alternative splicing in COL1A1 mRNA leads to a partial null allele and two In-frame forms with structural defects in non-lethal osteogenesis imperfecta. J Biol Chem 1996;271:28617-23. [Crossref] [PubMed]
- Molyneux K, Starman BJ, Byers PH, et al. A single amino acid deletion in the alpha 2(I) chain of type I collagen produces osteogenesis imperfecta type III. Hum Genet 1993;90:621-8. [Crossref] [PubMed]
- Oliver JE, Thompson EM, Pope FM, et al. Mutation in the carboxy-terminal propeptide of the Pro alpha 1(I) chain of type I collagen in a child with severe osteogenesis imperfecta (OI type III): possible implications for protein folding. Hum Mutat 1996;7:318-26. [Crossref] [PubMed]
- Pruchno CJ, Cohn DH, Wallis GA, et al. Osteogenesis imperfecta due to recurrent point mutations at CpG dinucleotides in the COL1A1 gene of type I collagen. Hum Genet 1991;87:33-40. [Crossref] [PubMed]
- Lund AM, Schwartz M, Skovby F. Variable clinical expression in a family with OI type IV due to deletion of three base pairs in COL1A1. Clin Genet 1996;50:304-9. [Crossref] [PubMed]
- Marini JC, Grange DK, Gottesman GS, et al. Osteogenesis imperfecta type IV. Detection of a point mutation in one alpha 1 (I) collagen allele (COL1A1) by RNA/RNA hybrid analysis. J Biol Chem 1989;264:11893-900. [PubMed]
- Marini JC, Lewis MB, Chen K. Moderately severe osteogenesis imperfecta associated with substitutions of serine for glycine in the alpha 1 (I) chain of type I collagen. Am J Med Genet 1993;45:241-5. [Crossref] [PubMed]
- Farber CR, Reich A, Barnes AM, et al. A novel IFITM5 mutation in severe atypical osteogenesis imperfecta type VI impairs osteoblast production of pigment epithelium-derived factor. J Bone Miner Res 2014;29:1402-11. [Crossref] [PubMed]
- Semler O, Garbes L, Keupp K, et al. A mutation in the 5'-UTR of IFITM5 creates an in-frame start codon and causes autosomal-dominant osteogenesis imperfecta type V with hyperplastic callus. Am J Hum Genet 2012;91:349-57. [Crossref] [PubMed]
- Cho TJ, Lee KE, Lee SK, et al. A single recurrent mutation in the 5'-UTR of IFITM5 causes osteogenesis imperfecta type V. Am J Hum Genet 2012;91:343-8. [Crossref] [PubMed]
- Balasubramanian M, Pollitt RC, Chandler KE, et al. CRTAP mutation in a patient with Cole-Carpenter syndrome. Am J Med Genet A 2015;167A:587-91. [Crossref] [PubMed]
- Amor IM, Rauch F, Gruenwald K, et al. Severe osteogenesis imperfecta caused by a small in-frame deletion in CRTAP. Am J Med Genet A 2011;155A:2865-70. [Crossref] [PubMed]
- Willaert A, Malfait F, Symoens S, et al. Recessive osteogenesis imperfecta caused by LEPRE1 mutations: clinical documentation and identification of the splice form responsible for prolyl 3-hydroxylation. J Med Genet 2009;46:233-41. [Crossref] [PubMed]
- Cabral WA, Chang W, Barnes AM, et al. Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat Genet 2007;39:359-65. [Crossref] [PubMed]
- van Dijk FS, Nesbitt IM, Zwikstra EH, et al. PPIB mutations cause severe osteogenesis imperfecta. Am J Hum Genet 2009;85:521-7. [Crossref] [PubMed]
- Christiansen HE, Schwarze U, Pyott SM, et al. Homozygosity for a missense mutation in SERPINH1, which encodes the collagen chaperone protein HSP47, results in severe recessive osteogenesis imperfecta. Am J Hum Genet 2010;86:389-98. [Crossref] [PubMed]
- Kelley BP, Malfait F, Bonafe L, et al. Mutations in FKBP10 cause recessive osteogenesis imperfecta and Bruck syndrome. J Bone Miner Res 2011;26:666-72. [Crossref] [PubMed]
- Alanay Y, Avaygan H, Camacho N, et al. Mutations in the gene encoding the RER protein FKBP65 cause autosomal-recessive osteogenesis imperfecta. Am J Hum Genet 2010;86:551-9. [Crossref] [PubMed]
- Shaheen R, Al-Owain M, Faqeih E, et al. Mutations in FKBP10 cause both Bruck syndrome and isolated osteogenesis imperfecta in humans. Am J Med Genet A 2011;155A:1448-52. [Crossref] [PubMed]
- Barnes AM, Cabral WA, Weis M, et al. Absence of FKBP10 in recessive type XI osteogenesis imperfecta leads to diminished collagen cross-linking and reduced collagen deposition in extracellular matrix. Hum Mutat 2012;33:1589-98. [Crossref] [PubMed]
- Puig-Hervás MT, Temtamy S, Aglan M, et al. Mutations in PLOD2 cause autosomal-recessive connective tissue disorders within the Bruck syndrome--osteogenesis imperfecta phenotypic spectrum. Hum Mutat 2012;33:1444-9. [Crossref] [PubMed]
- Steinlein OK, Aichinger E, Trucks H, et al. Mutations in FKBP10 can cause a severe form of isolated Osteogenesis imperfecta. BMC Med Genet 2011;12:152. [Crossref] [PubMed]
- Seyedhassani SM, Hashemi-Gorji F, Yavari M, et al. Novel FKBP10 Mutation in a Patient with Osteogenesis Imperfecta Type XI. Fetal Pediatr Pathol 2016;35:353-8. [Crossref] [PubMed]
- Lapunzina P, Aglan M, Temtamy S, et al. Identification of a frameshift mutation in Osterix in a patient with recessive osteogenesis imperfecta. Am J Hum Genet 2010;87:110-4. [Crossref] [PubMed]
- Martínez-Glez V, Valencia M, Caparrós-Martín JA, et al. Identification of a mutation causing deficient BMP1/mTLD proteolytic activity in autosomal recessive osteogenesis imperfecta. Hum Mutat 2012;33:343-50. [Crossref] [PubMed]
- Cho SY, Asharani PV, Kim OH, et al. Identification and in vivo functional characterization of novel compound heterozygous BMP1 variants in osteogenesis imperfecta. Hum Mutat 2015;36:191-5. [Crossref] [PubMed]
- Lv F, Xu XJ, Wang JY, et al. Two novel mutations in TMEM38B result in rare autosomal recessive osteogenesis imperfecta. J Hum Genet 2016;61:539-45. [Crossref] [PubMed]
- Asharani PV, Keupp K, Semler O, et al. Attenuated BMP1 function compromises osteogenesis, leading to bone fragility in humans and zebrafish. Am J Hum Genet 2012;90:661-74. [Crossref] [PubMed]
- Homan EP, Lietman C, Grafe I, et al. Differential effects of collagen prolyl 3-hydroxylation on skeletal tissues. PLoS Genet 2014;10:e1004121. [Crossref] [PubMed]
- Forlino A, Cabral WA, Barnes AM, et al. New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol 2011;7:540-57. [Crossref] [PubMed]
- Zayzafoon M. Calcium/calmodulin signaling controls osteoblast growth and differentiation. J Cell Biochem 2006;97:56-70. [Crossref] [PubMed]
- Glorieux FH, Rauch F, Plotkin H, et al. Type V osteogenesis imperfecta: a new form of brittle bone disease. J Bone Miner Res 2000;15:1650-8. [Crossref] [PubMed]
- Becker J, Semler O, Gilissen C, et al. Exome sequencing identifies truncating mutations in human SERPINF1 in autosomal-recessive osteogenesis imperfecta. Am J Hum Genet 2011;88:362-71. [Crossref] [PubMed]
- Rauch F, Husseini A, Roughley P, et al. Lack of circulating pigment epithelium-derived factor is a marker of osteogenesis imperfecta type VI. J Clin Endocrinol Metab 2012;97:E1550-6. [Crossref] [PubMed]
- Belinsky GS, Sreekumar B, Andrejecsk JW, et al. Pigment epithelium-derived factor restoration increases bone mass and improves bone plasticity in a model of osteogenesis imperfecta type VI via Wnt3a blockade. FASEB J 2016;30:2837-48. [Crossref] [PubMed]
- Symoens S, Malfait F, D'Hondt S, et al. Deficiency for the ER-stress transducer OASIS causes severe recessive osteogenesis imperfecta in humans. Orphanet J Rare Dis 2013;8:154. [Crossref] [PubMed]
- van Dijk F S, Zillikens M C, Micha D, et al. PLS3 mutations in X-linked osteoporosis with fractures. N Engl J Med 2013;369:1529-36. [Crossref] [PubMed]
- Daivajna S, Jones A, Hossein MS. Surgical management of severe cervical kyphosis with myelopathy in osteogenesis imperfecta: a case report. Spine (Phila Pa 1976) 2005;30:E191-4. [Crossref] [PubMed]
- Abelin K, Vialle R, Lenoir T, et al. The sagittal balance of the spine in children and adolescents with osteogenesis imperfecta. Eur Spine J 2008;17:1697-704. [Crossref] [PubMed]
- Primorac D, Rowe DW, Mottes M, et al. Osteogenesis imperfecta at the beginning of bone and joint decade. Croat Med J 2001;42:393-415. [PubMed]
- Watanabe G, Kawaguchi S, Matsuyama T, et al. Correlation of scoliotic curvature with Z-score bone mineral density and body mass index in patients with osteogenesis imperfecta. Spine (Phila Pa 1976) 2007;32:E488-94. [Crossref] [PubMed]
- Chen F, Guo R, Itoh S, et al. First mouse model for combined osteogenesis imperfecta and Ehlers-Danlos syndrome. J Bone Miner Res 2014;29:1412-23. [Crossref] [PubMed]
- Widhe TL. A probable new type of osteopenic bone disease. Pediatr Radiol 2002;32:447-51. [Crossref] [PubMed]
- Ivo R, Fuerderer S, Eysel P. Spondylolisthesis caused by extreme pedicle elongation in osteogenesis imperfecta. Eur Spine J 2007;16:1636-40. [Crossref] [PubMed]
- Rauch F. Material matters: a mechanostat-based perspective on bone development in osteogenesis imperfecta and hypophosphatemic rickets. J Musculoskelet Neuronal Interact 2006;6:142-6. [PubMed]
- Cabral WA, Makareeva E, Colige A, et al. Mutations near amino end of alpha1(I) collagen cause combined osteogenesis imperfecta/Ehlers-Danlos syndrome by interference with N-propeptide processing. J Biol Chem 2005;280:19259-69. [Crossref] [PubMed]


