Spinal subdural hematoma post foramen magnum decompression—rare complication in a patient with rhomboencephalosynapsis
Introduction
Spinal subdural hematomas (SSDH) represent less than 5% of all spinal hematomas (1). As a complication of posterior fossa surgery, SSDH is extremely rare (2,3) and only a few cases following cranial surgery have been reported (4-9). We report a case of delayed extensive SSDH presenting as cauda equina syndrome (CES) following foramen magnum decompression (FMD) and occipito-cervical (OC) fusion.
Case presentation
A 47-year-old woman was seen in outpatients with a 3-year history of worsening neck pain radiating to her left shoulder and upper arm. This was associated with mild to moderate upper cervical myeloradiculopathy which was gradually progressing.
Imaging of her neck revealed a Klippel-Feil abnormality with congenital fusion of the upper cervical vertebrae and abnormal articulations around the cranio-cervical junction (Figure 1A,B). It also showed significant basilar invagination with indentation of the medulla and upper cervical cord without definitive intrinsic cord signal changes. There was severe narrowing of the foramen magnum and marked upper cervical scoliosis resulting in significant foraminal narrowing on the left at C3/4, C4/5 and C5/6. Magnetic resonance imaging (MRI) of her brain showed absent formation of the vermis with continuity of the cerebellar hemispheres in keeping with rhomboencephalosynapsis. It also showed moderate chronic ventriculomegaly without evidence of acute obstruction.
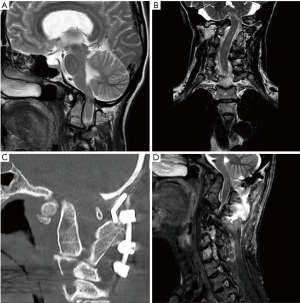
She underwent an elective FMD and occipito-cervical fusion (OC-C2-C3) with the aim to provide adequate neuronal decompression in order to prevent further deterioration and re-establish normal flow of cerebrospinal fluid (CSF) at the craniocervical junction (Figure 1C,D). Standard FMD including C1 laminectomy was performed with dura and arachnoid membranes dissected. An occipito-cervical fixation with occipital plate and screw-rod construct at C2 & C3 was inserted. The procedure was uneventful. Post-operatively the patient developed swallowing difficulties due to pharyngeal edema which was treated with a short course of oral steroids and speech and language therapy input. She was discharged home 8 days following her operation and at discharge her radiculopathy had resolved, her swallowing function had recovered and she was mobilizing independently.
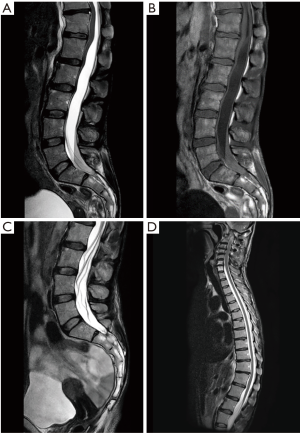
She represented 2 days later (post-operative day 10) with severe lower back pain, bilateral sciatica and urinary retention. Her symptoms had started just after discharge and gradually deteriorated over 48 hours. On examination she had 4/5 power in both lower limbs associated with painful paresthesia in her lower limbs and altered perineal sensation. An MRI scan of her spine revealed a large intradural posterior collection of blood and fluid which was anteriorly displacing and compressing the conus medullaris and cauda equina at the lumbo-sacral region (Figure 2A,B). Laboratory evaluation revealed no evidence of coagulopathy. She was managed with serial lumbar punctures (×3) with large amounts (>20 mL) of CSF drained each time. The initial CSF samples were blood stained (altered blood) and the first sample had a red cell count of 16,400. Symptoms and signs improved over a course of 5 days after clinical onset and the patient made a complete neurological recovery. Follow-up MRI demonstrated delayed radiological improvement as well (Figure 2C,D).
Discussion
A large SSDH as a complication of surgery at the craniocervical junction or posterior fossa is extremely rare. The source of bleeding in SSDH is unclear (8). Apart from the artery of Adamkiewicz in the thoracolumbar region, the spinal subdural space does not contain major blood vessels or bridging veins (10). Therefore, unlike intracranial subdural hematomas, the cause of SSDH is unlikely to be due to rupture of vessels along the undersurface of the dura (9). This explains, in part, why SSDH are not as common as cranial subdural hematomas. Several pathogenic mechanisms for SSDH have been suggested including a sudden rise in pressure within the spinal vessels due to an increase of pressure in the abdominal or thoracic cavity (11,12). Known etiological factors for non-traumatic SSDH include coagulopathy, vascular malformation, lumbar puncture, spinal surgery and rarely ruptured spinal arachnoid cyst (8,12). Although, SSDH can also occur in the absence of these known risk factors.
Previous authors have suggested the formation of SSDH following cranial surgery may be due to migration of blood from the cranial subdural space to the spinal subdural space under the influence of gravity (4-10). Anatomically the cranial and spinal subdural spaces are thought to be in continuity (13) and in this case the layering of blood in the gravity-dependent lower areas of the spinal canal would support the theory of downward migration of blood. Lee and Hong (4) described six cases of SSDH which developed after surgery for intracranial lesions. In 4 of the 6 cases the initial cranial procedure was a temporal lobectomy which involved opening the temporal horn of the lateral ventricle resulting in large amounts of CSF drainage. They hypothesized CSF over drainage during cranial surgery potentially results in CSF hypotension in the spinal subarachnoid space and may aid migration of blood from the cranium to the spine (4).
In our case, large amount of CSF was not drained intra-operatively and not even a large pseudomeningocoele was created. Moreover, there was no hematoma in the cranial compartment to migrate into the spine. We presume that restoration of normal CSF flow at the cranio-cervical junction altered the hydrodynamics of the spinal CSF circulation and a change in the CSF pressure gradient possibly caused rupture of an undiagnosed pre-existing intradural spinal arachnoid cyst. An MRI of the whole spine was not obtained pre-operatively which is why this spinal arachnoid cyst was not identified in advance. Pre-operative screening included MRI of brain and cervical spine but not the thoracic or lumbar spine, which is where we presume the arachnoid cyst was located. Most intradural arachnoid cysts have been reported to be in the thoracic spine and rarely in the lumbar spine (12).
Onset of symptoms in this case was around the eighth post-operative day when the patient was discharged home. Previous authors have reported onset of symptoms 4 to 12 days after initial cranial surgery (4,5,7-9). MRI is the investigation of choice and in our case the MRI of the whole spine was suggestive of a lumbosacral hyperacute subdural hematoma as the collection was hypointense on T1 weighted images and hyperintense on T2 weighted images (14). The relative hypovascularity in the subdural space may have slowed down the metabolism of the hematoma which could explain the discrepancy between the clinical presentation (subacute clinical course, MRI done more than 2 days after symptom onset) and radiological diagnoses (hyperacute SSDH) (12).
There are no guidelines for the treatment of SSDH. We decided to manage our patient with serial lumbar punctures. She was admitted to a neurosurgical ward and closely observed for any progressive neurological deficit. If she had deteriorated neurologically decompressive spinal surgery and drainage of the hematoma would have been performed. Liu and colleagues (8) reviewed 22 published cases of SSDH and found 17 (77%) patients had been treated conservatively or with lumbar puncture and only 5 (23%) patients treated surgically. All but 1 of the 22 patients made a good neurological recovery.
Conclusions
SSDH as a complication of posterior fossa surgery is rare. The clinical features are related to mass effect of the hematoma on the spinal cord and cauda equina (15). The clinical course of SSDH post cranial surgery appears to be benign; most reported cases did not require surgical intervention and patients made a good neurological recovery. However, SSDH could have potentially serious consequences. We recommend early diagnosis using MRI imaging and close monitoring of neurological state. Prompt surgery should be performed in the presence of neurological deterioration but our experience revealed that serial therapeutic lumbar punctures can provide full neurological recovery.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Kreppel D, Antoniadis G, Seeling W. Spinal hematoma: a literature survey with meta-analysis of 613 patients. Neurosurg Rev 2003;26:1-49. [Crossref] [PubMed]
- Dubey A, Sung WS, Shaya M, et al. Complications of posterior cranial fossa surgery--an institutional experience of 500 patients. Surg Neurol 2009;72:369-75. [Crossref] [PubMed]
- Arnautovic A, Splavski B, Boop FA, et al. Pediatric and adult Chiari malformation Type I surgical series 1965-2013: a review of demographics, operative treatment, and outcomes. J Neurosurg Pediatr 2015;15:161-77. [Crossref] [PubMed]
- Lee JI, Hong SC. Spinal subdural haematoma as a complication of cranial surgery. Acta Neurochir (Wien) 2003;145:411-4; discussion 414-5. [PubMed]
- Kim MS, Chung CK, Hur JW, et al. Spinal subdural hematoma following craniotomy: case report. Surg Neurol 2004;61:288-92. [Crossref] [PubMed]
- Hicdonmez T, Kilincer C, Hamamcioglu MK, et al. Paraplegia due to spinal subdural hematoma as a complication of posterior fossa surgery: Case report and review of the literature. Clin Neurol Neurosurg 2006;108:590-4. [Crossref] [PubMed]
- Kim MS, Lee CH, Lee SJ, et al. Spinal subdural hematoma following intracranial aneurysm surgery: four case reports. Neurol Med Chir (Tokyo) 2007;47:22-5. [Crossref] [PubMed]
- Liu J, Wu B, Feng H, et al. Spinal subdural hematoma following cranial surgery: a case report and review of the literature. Neurol India 2011;59:281-4. [Crossref] [PubMed]
- Liao CH, Chang FC, Hsu SP, et al. Spinal subdural hematoma following posterior fossa surgery. Formosan J Surg 2013;46:52-5. [Crossref]
- Kanamaru H, Kanamaru K, Araki T, et al. Simultaneous Spinal and Intracranial Chronic Subdural Hematoma Cured by Craniotomy and Laminectomy: A Video Case Report. Case Rep Neurol 2016;8:72-7. [Crossref] [PubMed]
- Rader JP. Chronic subdural hematoma of the spinal cord: report of a case. N Engl J Med 1955;253:374-6. [Crossref] [PubMed]
- Huang KC, Chuang MT, Huang DW, et al. Lumbar intraspinal arachnoid cyst superimposed by hyperacute spinal subdural hematoma: an unusual case. Spine J 2011;11:e5-8. [Crossref] [PubMed]
- Lecouvet FE, Annet L, Duprez TP, et al. Uncommon magnetic resonance imaging observation of lumbar subdural hematoma with cranial origin. J Comput Assist Tomogr 2003;27:530-3. [Crossref] [PubMed]
- Manish KK, Chandrakant SK, Abhay MN. Spinal Subdural Haematoma. J Orthop Case Rep 2015;5:72-4. [PubMed]
- Treister DS, Kingston SE, Zada G, et al. Concurrent intracranial and spinal subdural hematoma in a teenage athlete: a case report of this rare entity. Case Rep Radiol 2014;2014:143408. [PubMed]


