A rare case of intramedullary solitary juvenile xanthogranuloma of the lumbar spine in the adult: a case report
Introduction
Solitary juvenile xanthogranuloma (SJX) is a rare proliferative histiocytic disorder (non-Langerhans histiocytosis) usually affecting children or young adults. Its etiology and pathogenesis are unknown. It mostly presents with self-resolving skin lesions (1). Lesions sited elsewhere, especially in the spine, are rare. Less than 15 cases of spinal XJS have been described so far in English-speaking literature (2,3). All the above cases were extramedullary, about half of which were diagnosed after the second decade of life. This case report describes the first intramedullary spinal SJX in a 22-year-old male patient.
Case presentation
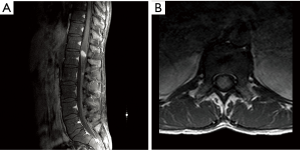
A 22-year-old male presented with a nine-month history of progressively worsening sphincteric disturbances and saddle hypoesthesia. Onset of symptoms was 9 months previous to our clinical evaluation. Magnetic resonance imaging was showed an intra-axial lesion located in the conus medullaris that was T1 hypointense, T2 iso-hyperintense and uniformly enhancing after contrast administration (Figure 1). The lesion was removed through a T12–L1 laminectomy and a median myelotomy with neurophysiological monitoring. The mass appeared as greyish and solid with no cleavage plane from the surrounding parenchyma, thus making it hard to achieve radical removal. The post-operative course was uneventful and the patient was discharged from hospital in seven days. The histology report described a histiocytic proliferative lesion with moderate/high cellularity and significative lymphoplasmacytic inflammatory infiltrate (Figure 2A). Histiocytes have an ovular or kidney-shaped appearance, with fine chromatin nuclei and eosinophilic cytoplasm with poorly defined margins. In these areas, gigantocellular multinucleated cells are found. The number of mitoses is not significantly increased and no apoptotic or necrotic areas are seen. The Ki67 proliferative index reaches a maximum of 16%. Immunohystochemical analysis shows diffuse positivity, especially in the lymphoplasmacellular infiltrate, to LCA, CD3, CD4, CD8 and to a lesser extent to CD20 and CD79. The histiocytic elements are intensely positive for macrophage markers such as CD68 and CD163, and for HLA-DR, CD 123, CD68R and vimentin. CD1a, CD21, CD35, tryptase and langerin were not found (Figure 2B). Positivity to glial fibrillary acidic protein (GFAP) and S100 is found only in pre-existing structures (Figure 2C,D). No positivity to CK-Lu5.
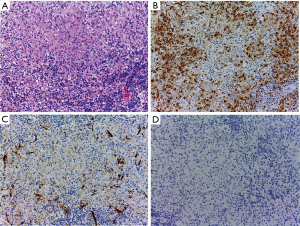
Considering its negativity to CD1a and S100 we can regard this lesion as non-Langerhans histiocytosis. Under a histological and immunohistochemical point of view this is compatible with a juvenile xanthogranuloma.
After positron emission tomography (PET) scan imaging, no pathological enhancement, apart from the chord nodule was noted. No secondary localizations after a full-body computed tomography (CT) scan (therefore ruling out Erdheim-Chester disease).
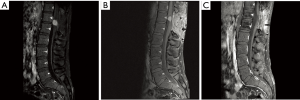
A spine MRI performed after 6 months displayed a 7-mm (diameter) residual lesion. The following imaging at 12 months showed a growth with a 21-mm (diameter) intramedullary recurrency (Figure 3A). The patient’s neurological examination showed a clear worsening involving the left lower-limb distal muscles, mostly upon his left foot, with sensory paresthesia and a mild motor deficit not compromising his ability to walk. After 18 months from the first surgical procedure, the patient underwent a new microsurgical, neurophysiology-assisted procedure with gross-total removal through the previous surgical approach. He was discharged from hospital on postoperative day ten. At a 3-month follow-up contrast-enhanced MRI, a neoplastic recurrent 1 cm mass was found and later confirmed by fludeoxyglucose (18F-FDG) CT scan. A new MRI after 2 months showed a volumetric increase to 1.5 cm (Figure 3B). The patient underwent a third procedure, followed by adjuvant focal radiotherapy. A planning CT in prone position in a blue bag (BodyFIX® system) was performed. A clinical target volume (CTV) structure was defined based on the initial preoperative extension of the lesion on MRI T1 with contrast and T2 sequence and including the resection cavity and adding a margin cranio-caudal at least of 10 and 20 mm. For the planning target volume (PTV) another margin of 5 mm in all directions was added. The treatment was performed with a fractionated schedule of 27×1.8 Gy and a total dose of 48.6 Gy. Daily positioning was checked with portal imaging. The latest follow-up radiological and clinical evaluations show no signs of recurrence (Figure 3C). He returned to his workplace and no further neurological deficits are currently present.
Discussion
SJX is a rare proliferative histiocytic disorder defined as non-Langerhans histiocytosis. Despite the controversial nature of these lesions, the presence of monoclonal cells leads to the hypothesis of neoplastic lesions (4). It most commonly affects children, with self-limiting skin lesions appearing mostly during the first two decades of life and in the male population. SJX can also appear during the adult life, mostly during the third and fourth decades, with no gender preponderance. SJX is prevalently a skin disorder, with systemic manifestations ranging between 5% and 10% of all cases. It most frequently affects the eyes. Other possible sites are the heart, liver, testes, oral mucosa and the central nervous system. Within the brain, the most commonly reported localization is intraparenchymal or intraventricular at the choroid plexus. Extracutaneous lesions involving the spine are rare (4,5). To the best of our knowledge, less than 15 cases of spinal SJX have been described in the English-speaking literature. Only 6 cases were found after the second decade of life. Spinal SJXs were found extradurally as osteolytic lesions within the vertebral body (2,3,6-15) or intradurally extramedullary involving the spinal and the cauda equina roots (16-18). All the above cases were extramedullary, of which six were diagnosed after the second decade of life. This case report describes the first intramedullary spinal SJX. Differential diagnoses, including ependymomas, astrocytomas and other rare tumors involving the intramedullary compartment, should be considered. On MRI, SJXs appear isointense on T1 and iso-hyperintense on the T2 weighted sequences with dishomogeneous contrast enhancement. Its macroscopic appearance is of an encapsulated mass with or without a cystic component, yellowish-grey in color. In our reported case, no cleavage plane was present. The histological examination displays foamy histiocytic cells with and without giant Touton cells upon a mononucleate-infiltrate background. After immunohistochemical analysis the giant mononucleated or fusiform cells show positivity for lysosomal staining and an immunoreactivity to vimentin, CD68, CD163, fascin, CD14 and factor XIIIa but negativity for CD1A, a typical Langerhans cell marker, and for S-100 protein (present in the Rosai-Dorfmann disease) (19).
Skin lesions in children have a tendency to spontaneous regression in a period of months or years and fade away in the young adult, therefore requiring only clinical monitoring. In the adult population such regression is not commonly described. As opposed to skin lesions, no cases of spontaneously-regressing spinal SJX localizations were noted. These required treatment when symptomatic. Given the rarity of these tumors, no consensus is present for the optimal treatment strategy, even if surgical removal has been the elective therapeutic approach for all reported cases in the literature. Radical removal has shown to be curative and not requiring adjuvant treatment. The case described in this paper differs from the common description of SJX as a benign lesion. The intramedullary location led to a higher morbidity after surgery. The infiltrative characteristics, in the absence of a proper tumor sheath, did not allow radical removal after the first surgical approach. The timing of recurrency and the volumetric increase we noticed after radiological imaging lean towards a relatively fast growth. Finally, similarly to malignant tumors, the above-reported SJX was treated with adjuvant radiotherapy, achieving remission at a 2-year follow-up.
Conclusions
In our reported case, due to the intramedullary localization and the absence of a clear cleavage plane, radical removal was not possible. The tumor subsequently recurred and new surgical procedures were necessary. In accordance to what has been reported so far in the literature, SJX bears a high risk of recurrency and its radical removal, or subtotal removal followed by adjuvant radiotherapy, are the treatment of choice.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Hernandez-Martin A, Baselga E, Drolet BA, et al. Juvenile xanthogranuloma. J Am Acad Dermatol 1997;36:355-67. [Crossref] [PubMed]
- Bhaisora KS, Jaiswal AK, Mehrotra A, et al. Solitary juvenile xanthogranuloma of the cervical spine in a child: A case report and review of literature. Asian J Neurosurg 2015;10:57. [Crossref] [PubMed]
- Purohit D, Chanduka AK, Sharma V, et al. Juvenile Xanthogranuloma of adult spine: A rare case and review of literature. Asian J Neurosurg 2014;9:239. [PubMed]
- Janssen D, Harms D. Juvenile xanthogranuloma in childhood and adolescence: a clinicopathologic study of 129 patients from the kiel pediatric tumor registry. Am J Surg Pathol 2005;29:21-8. [Crossref] [PubMed]
- Dehner LP. Juvenile xanthogranulomas in the first two decades of life: a clinicopathologic study of 174 cases with cutaneous and extracutaneous manifestations. Am J Surg Pathol 2003;27:579-93. [Crossref] [PubMed]
- Agabegi SS, Iorio TE, Wilson JD, et al. Juvenile xanthogranuloma in an adult lumbar spine: a case report. Spine 2011;36:E69-73. [Crossref] [PubMed]
- Cao D, Ma J, Yang X, et al. Solitary juvenile xanthogranuloma in the upper cervical spine: case report and review of the literatures. Eur Spine J 2008;17 Suppl 2:S318-23. [Crossref] [PubMed]
- Fulkerson DH, Luerssen TG, Hattab EM, et al. Long-term follow-up of solitary intracerebral juvenile xanthogranuloma. Case report and review of the literature. Pediatr Neurosurg 2008;44:480-5. [Crossref] [PubMed]
- Inoue H, Seichi A, Yamamuro K, et al. Dumbbell-type juvenile xanthogranuloma in the cervical spine of an adult. Eur Spine J 2011;20 Suppl 2:S343-7. [Crossref] [PubMed]
- Jain A, Mathur K, Khatri S, et al. Rare presentation of juvenile xanthogranuloma in the thoracic spine of an adult patient: case report and literature review. Acta Neurochir (Wien) 2011;153:1813-8. [Crossref] [PubMed]
- Konar S, Pandey P, Yasha TC. Solitary juvenile xanthogranuloma in cervical spine: case report and review of the literature. Turk Neurosurg 2014;24:102-7. [PubMed]
- Lee SJ, Jo DJ, Lee SH, et al. Solitary xanthogranuloma of the upper cervical spine in a male adult. J Korean Neurosurg Soc 2012;51:54-8. [Crossref] [PubMed]
- Rampini PM, Alimehmeti RH, Egidi MG, et al. Isolated cervical juvenile xanthogranuloma in childhood. Spine 2001;26:1392-5. [Crossref] [PubMed]
- Shimosawa S, Tohyama K, Shibayama M, et al. Spinal xanthogranuloma in a child: case report. Surg Neurol 1993;39:138-42. [Crossref] [PubMed]
- Wille DA, Bozinov O, Scheer I, et al. Isolated intraspinal juvenile xanthogranuloma in an infant presenting as acute paraplegia. Neuropediatrics 2013;44:171-3. [PubMed]
- Castro-Gago M, Gómez-Lado C, Alvez F, et al. Juvenile xanthogranuloma of the cauda equina. Pediatr Neurol 2009;40:123-5. [Crossref] [PubMed]
- Iwasaki Y, Hida K, Nagashima K. Cauda equina xanthogranulomatosis. Br J Neurosurg 2001;15:72-3. [Crossref] [PubMed]
- Kitchen ND, Davies MS, Taylor W. Juvenile xanthogranuloma of nerve root origin. Br J Neurosurg 1995;9:233-7. [Crossref] [PubMed]
- Haroche J, Abla O. Uncommon histiocytic disorders: Rosai-Dorfman, juvenile xanthogranuloma, and Erdheim-Chester disease. Hematology Am Soc Hematol Educ Program 2015;2015:571-8. [Crossref] [PubMed]


