Spinal metastases of two different grade oligodendrogliomas: a case report and review of literature
Introduction
Oligodendrogliomas (OGD) are glial tumors and together with mixed oligoastrocytomas, constitute 5-20% of all gliomas (1). They occur predominantly in adults, with a peak between 40 and 60 years of age. Low-grade OGD tend to arise in younger patients, as do all grade II and III tumors with isocitrate dehydrogenase (IDH) mutations with and without 1p/19q co-deletion (1,2). Since the discovery of 1p/19q co-deleted oligodendroglial tumors being sensitive to chemotherapy, surgical resection with chemotherapy have become the mainstays of treatment. Recent molecular studies have shown that all 1p/19q co-deleted tumors have IDH mutations and most of them also have telomerase reverse transcriptase (TERT) mutations, which has led to fundamental considerations of how oligodendroglial tumors should be defined: by histopathology, by the presence or absence of specific molecular lesions or by a combination of these two (1).
Typically, these tumors arise in the white matter of cerebral hemispheres, and are located supratentorially (85%), with the frontal lobe being the most common location (50–65%) (2), and are generally round to oval appearing as a relatively well-circumscribed mass of variable size. In shape it resembles low-grade diffuse astrocytoma, but OGD, in contrast to diffuse astrocytoma, has a characteristic affinity for the cortex (3) - it is typically superficially located, involving both the cortex and the subcortical area, so patients can present nonspecific signs according to localization and the rate of tumor progression. However, low-grade OGD tend to cause seizures many years before other symptoms arise. In contrast, patients with high-grade tumors present with focal deficits, signs of increased intracranial pressure or cognitive deficits earlier in the course of their disease (1).
On CT, OGD are typically hypodense, but heterogeneity of density (mixed hypo-isodensity) is also commonly seen. Signal intensity on magnetic resonance imaging (MRI) is generally lower than that of grey matter on T1 weighted sequences. On T2 weighted imaging the tumor is hyperintense with commonly marked heterogeneity and with intravenous contrast they generally do not enhance. Minimal to moderate patchy, multifocal enhancement with a dot-like or lacy pattern is, however, reported in up to 50% of cases. This distinguishes OGD from other low-grade gliomas that do not enhance, and has been suggested to be more common in mixed oligoastrocytoma than in OGD (2,4). The most important differential diagnosis of OGD is anaplastic OGD, though these tumors of different World Health Organization (WHO) grades cannot be reliably distinguished on conventional imaging.
Lesions at the spinal cord frequently occur after surgical treatment of the primary tumor, even many years after their diagnosis. Most leptomeningeal metastases from primary brain tumors are from malignant high-grade tumors such as glioblastomas, anaplastic gliomas, and medulloblastomas. For malignant astrocytic gliomas, dissemination through the cerebrospinal fluid has been described at 10–21% at autopsy (5,6). the presence of these new lesions become a diagnostic and therapeutic challenge. The cases reporting metastases secondary to low grade gliomas demonstrations demonstrate the recurrence of the tumors at the primary site of resection (5).
Case presentation
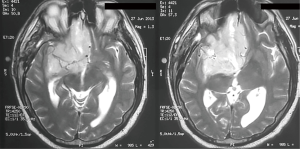
We present the case of a male patient of 46 years old without history of disease, who presented to the emergency department for 20 days of severe headache, confusion and vomiting, with an MRI that shows a neoplastic lesion in the right medial fronto-basal region of great size and heterogeneous components (cyst and solid). The lesion displaces the midline more than 10 mm with contrast uptake and perilesional edema (Figure 1).
The Patient was taken to surgery and was found to have a right frontal subcortical infiltrative neoplastic lesion with infiltration to the Sylvian fissure, with cleavage planes, bland, many blood vessels, rubbery areas, necrotic pinkish and yellow areas.
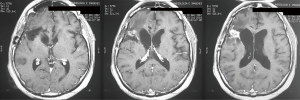
The surgery was complete without any complications. MRI post-surgery indicated complete resection of the tumor, with no hemorrhagic or ischemic process (Figure 2).
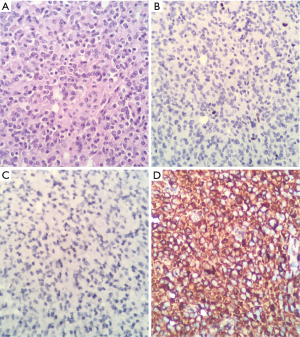
Final report of pathology and immunohistochemistry indicates: oligodendroglioma WHO grade II, KI67: 2–3% (Figure 3).
According to the oncology department, the patient received oral chemotherapy with Temozolamida. Two months after surgery, radiotherapy was commenced with 50 g doses.
Treatment was ended 4 months after diagnosis with appropriate clinical and imaging being performed.
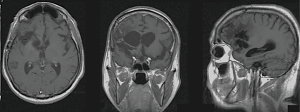
Two years after the diagnosis, he was involved in a traffic accident. He presented with progressive asymmetric motor paraparesis −2/5 and loss of sphincter control caused by acute urinary retention and the absence of bowel movements. Spine MRI showed an intra-axial and an expansive intramedullary lesion at the level of the medullary cone and vertebral bodies T12 and L1, poorly defined and regular contours approximately 5.1×1.7 cm, hypointense on T1 and hyperintense on T2 sequences with central intramedullary hyperintensity extending through the ependimary channel from T6 to T11 that is probably related to edema (Figure 4).

The cerebral studies demonstrate that no sign of residual tumor was found and on this premise we discarded the possibility of current tumor recurrence and metastasis secondary to recurrent tumor (Figure 5).
Therefore, we considered medullary compression syndrome and emergency surgery was performed, which revealed a neoplastic lesion with extramedullary compressive strength on the conus medullary and wrapping all of the roots of the proximal part. Also observed were violet with white hypervascularized areas with occasional areas of cleavage but with marked shift on the medullary cone from left to right. Final report of pathology and immunohistochemistry indicates OGD WHO grade III, KI67: 80% (Figure 6).
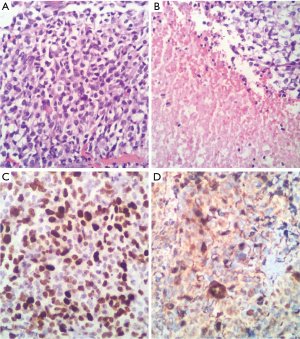
After surgery, the patient demonstrated progressive improvement of strength and sensitivity of the lower limbs. According to oncology department, the patient received oral chemotherapy and radiotherapy.
Discussion
Gliomas originate from neural stem cells or glial progenitor cells that develop or maintain glial characteristics, and diffuse gliomas are by far the most frequent neoplasms originating from the CNS parenchyma itself. The common denominator of this heterogeneous group of neoplasms is extensive, infiltrative growth of individual tumor cells and/or groups of these cells (‘diffuse growth’) into the neuropil (7). OGD and oligoastrocytomas have been generally grouped together as oligodendroglial tumors and account for less than 10% of the diffuse gliomas. According to WHO 2016 diagnostic classification, OGD and anaplastic OGD requires the demonstration of both an IDH gene family mutation and combined whole-arm losses of 1p and 19q (1p/19q codeletion) (8).
As previously mentioned, OGD occurs predominantly in adults, with a peak incidence between the fourth and sixth decade of life. Although median survival in patients with low-grade OGD may be more than ten years, their location makes a surgical approach complicated, which lowers life expectancy as a result of associated surgical risks. Progress has been made with OGD patients such that the current standard of care (surgery, radiotherapy and chemotherapy) yields median survival times of ten to fourteen years even in WHO grade III examples, while patients with a WHO grade II OGD may survive even longer (7), with evidence showing that surgery that maximises resection margins as objectively measured on postoperative MRI demonstrates increased overall survival as a result of delayed malignant transformation. This together with the use of procarbazine, lomustine and vincristine (PCV) chemotherapy and temozolomide could extend the survival of some patients (9).
Rarely, extracranial metastases of oligodendroglial tumors occur (10) and only a tiny fraction of this tend to be due to single-gene hereditary syndromes such as biallelic Lynch síndrome (11). The most common glioma to metastasize is glioblastoma multiforme, followed by medulloblastoma and ependymoma, while OGD very rarely metastasizes (12). Primary neoplasms in the brain is generally considered to spread in any of the following three ways: seeding through the cerebral fluid pathway, local invasion or remote spread through lymphatic, blood vessels and surgical procedures (13). Metastasis at the spine axis mainly occurs as diffuse microscopic subarachnoid spreading, whereas spinal cord localizations are very rare and has been reported mainly in patients with glioblastoma and anaplastic astrocytoma (up to 20% of cases with CSF positivity) (14), it is also rare for OGD (8.5-14% of CSF microscopic seeding) (15). In this situation, tumour cells are thought to gain access to the CSF space by breaching the ependyma or pia mater (3,4). This phenomenon of diffuse CSF spread of primary central nervous system tumours is well recognised in primitive neuroectodermal tumors such as medulloblastomas and in various gliomas (14).
However, symptomatic involvement of the spinal cord from brain oligodendroglioma is very rare. According to Elefante et al., only 16 cases were reported in 2012 (10). A review of the literature by Li et al. in 2014 using the PubMed search terms “extracranial”, ‘extraneural’, ‘oligodendroglioma’, ‘OGD”, “metastatic’, ‘metastasis’, and ‘metastases’ shows 61 reportedmetastatic OGDs of which 33 were male (54.1%), 17 were female (27.9%), - in the remaining 18.0%, gender was not reported. There were 110 infiltrated sites correlated closely with primary OGDs. The most frequent metastatic site was bone and bone marrow (n=47; 42.7%) followed by lymph nodes (n=22; 20.0%), liver (n=7; 6.4%), scalp (n=6; 5.5%), lung (n=6; 5.5%), pleura (n=4; 3.6%), chest wall (n=3; 2.7%), iliopsoas muscle (n=2; 1.8%), soft tissue (n=2; 1.8%) parotid gland (n=2; 1.8%), and adrenal gland, spleen, thoracic wall, pancreas, dorsal root ganglia, abdomen, spinal dura mater, breast and thymus gland with one lesion each (n=1; 0.9%) (16). This confirms the worldwide rarity of metastasis.
At the time of this case report, using the following PubMed search terms: ‘grade II and III OGD at the same patient,’ ‘metastatic,’ and ‘metastases,’ we did not find any matches available. However, in our country the availability of pathology laboratories and qualified personnel is limited to the evaluation of genetic markers that confirm the two pathologies, which limits the ability to consider it to be the first reported case. At the moment this is the 18th case of low-grade glioblastoma with symptomatic spinal metastasis.
Conclusions
OGD with symptomatic spinal metastasis is a very rare event with less than 20 cases reported to date, thus there is no standardized treatment.
This generates significant questions: are there other cases in which a single patient has two different grades of OGD? Does this predispose to new and more aggressive metastasis considering that the patient was taken to two surgeries on the neuraxis? After performing the review as mentioned above, we did not find another patient with two different grades of the same tumor. On follow-up two years following his last surgery, the patient is in good condition. Imaging performed every six months confirms no new metastasis.
From our experience, MRI of the neuraxis in patients who have undergone surgery for any grade of glioma is useful in detecting early spinal metastasis. We make a respectful call to all neurosurgeons who may have access to our report and have patients with metastases of two different grade of gliomas to share their experience with our department of neurosurgery in Foscal Clinic, Colombia.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflict of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report.
References
- van den Bent MJ. Anaplastic oligodendroglioma and oligoastrocytoma. Neurol Clin 2007;25:1089-109. ix-x. [Crossref] [PubMed]
- Smits M. Imaging of oligodendroglioma. Br J Radiol 2016;89:20150857. [Crossref] [PubMed]
- Osborn A. Osborn's Brain: Imaging, pathology and anatomy. Osborn's Brain: Imaging, pathology and anatomy. Amirsys, Inc.; 2012.
- Koeller KK, Rushing EJ. From the archives of the AFIP: Oligodendroglioma and its variants: radiologic-pathologic correlation. Radiographics 2005;25:1669-88. [Crossref] [PubMed]
- Moore MT, Eisinger G. Extra primary seeding of glioblastoma multiforme in the subarachnoid space and ependyma. Neurology 1963;13:855-65. [Crossref] [PubMed]
- Verma N, Nolan C, Hirano M, et al. Intramedullary spinal cord and leptomeningeal metastases from intracranial low-grade oligodendroglioma. Clin Imaging 2014;38:505-7. [Crossref] [PubMed]
- Wesseling P, van den Bent M, Perry A. Oligodendroglioma: pathology, molecular mechanisms and markers. Acta Neuropathol 2015;129:809-27. [Crossref] [PubMed]
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803-20. [Crossref] [PubMed]
- Ellenbogen JR, Davies P, Eldridge PR, et al. Unusual patterns of recurrence in low grade gliomas. J Clin Neurosci 2014;21:360-3. [Crossref] [PubMed]
- Elefante A, Peca C, Del Basso De Caro ML, et al. Symptomatic spinal cord metastasis from cerebral oligodendroglioma. Neurol Sci 2012;33:609-13. [Crossref] [PubMed]
- Garner J, Morcos Y, Bari M. Extradural cord compression due to metastatic oligodendroglioma. J Neurooncol 2002;58:71-5. [Crossref] [PubMed]
- Han SR, Yoon SW, Yee GT, et al. Extraneural metastases of anaplastic oligodendroglioma. J Clin Neurosci 2008;15:946-9. [Crossref] [PubMed]
- Gyepes MT, D'angio GJ. Extracranial metastases from central nervous system tumors in children and adolescents. Radiology 1966;87:55-63. [Crossref] [PubMed]
- Choucair AK, Levin VA, Gutin PH, et al. Development of multiple lesions during radiation therapy and chemotherapy in patients with gliomas. J Neurosurg 1986;65:654-8. [Crossref] [PubMed]
- Earnest F 3rd, Kernohan JW, Craig WM. Oligodendrogliomas; a review of 200 cases. Arch Neurol Psychiatry 1950;63:964-76. [Crossref] [PubMed]
- Li G, Zhang Z, Zhang J, et al. Occipital anaplastic oligodendroglioma with multiple organ metastases after a short clinical course: a case report and literature review. Diagn Pathol 2014;9:17. [Crossref] [PubMed]