CT guided radiofrequency ablation of the cervical medial branch using a lateral approach in the supine patient
Introduction
The facet joints are a common source of cervicalgia, implicated in up to 55% of patients with chronic neck pain, including following whiplash injury and cervical spine surgery (1-5).
Therapeutic techniques for the management of chronic facet joint pain include facet joint denervation via radiofrequency ablation of the medial branch of the spinal dorsal ramus. The efficacy of the procedure was initially evaluated in a double blind controlled trial by Lord et al. and has been further validated in several observational and prospective studies (6-9).
Traditionally medial branch denervation has been performed under fluoroscopic guidance, however the advent of CT fluoroscopy has provided an alternative modality for image guided intervention. To our knowledge the technique and anatomical landmarks used for CT guided medial branch denervation have not previously been described.
This article describes the technique for CT guided radiofrequency denervation of the cervical medial branch using a lateral approach in a supine patient.
Anatomy and CT correlation
Each facet joint is innervated by the medial branch of the dorsal ramus of the cervical spinal nerve from above and below. For instance, the C5/6 facet joint is innervated by the medial branches of both C5 and C6.
After exiting the neural foramina, each cervical spinal nerve divides into ventral and dorsal rami. The dorsal ramus subsequently divides into medial and lateral branches, with the medial branch coursing around the lateral margin of the articular pillar to its dorsal aspect (Figure 1A,B).
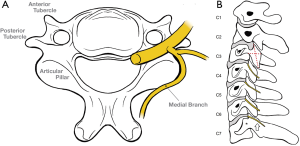
Innervation of the upper and lower cervical facet joints differs slightly from the mid-cervical spine (discussed below):
C3 to C6 levels
Anatomic studies have demonstrated that the course of the C3–6 medial branches pass through the midpoint of the trapezoidal shape formed by the articular pillar on lateral neck imaging. This is a recognised landmark for medial branch denervation performed using fluoroscopy (Figure 1B).
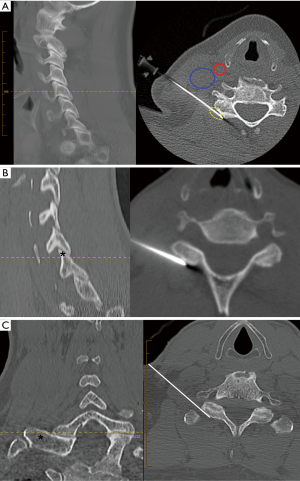
Utilizing multi-planar CT reconstruction, the traditional fluoroscopic landmark for the C3-6 levels correlates to the most cranial axial CT slice where both the posterior tubercle of the transverse process and articular pillar are visible (Figure 2A). The target needle tip position is the dorsolateral cortex of the articular pillar.
C7 to C8 levels
The C7 medial branch is elevated by the large C7 transverse process and thus courses high towards the apex of the superior articular process (Figure 1B).
The C8 medial branch traverses laterally along the superior cortex of the transverse process. The articular branches to the facet joint arise from the medial branch proximally near the base of the transverse process.
For C7 and C8 medial branch denervation, the axial CT target level is the slice immediately cranial to the superior cortex of the transverse process (Figure 2B,C). The target needle tip position is the dorsolateral cortex of the superior articular process at the junction with the transverse process.
C2 Level
The C2/3 facet joint is supplied primarily via the third occipital nerve (TON). Discussion of CT guided denervation of the TON is beyond the scope of this paper.
Technique
To minimise patient motion, we perform the procedure under conscious sedation administered by an anaesthetist. The patient is positioned supine on the CT scanner table. A cushion is used to elevate the head, placing the cervical spine into a neutral position minimising flexion, extension, or rotation. This standardised neck position aims to ensure constancy and reproducibility of the bony target landmarks. The patient’s head and arms are stabilised. The dispersive electrode pad (DGP-PM, Cosman Medical, Burlington, Massachusetts, USA) is secured to the patient’s anterolateral thigh.
Sagittal scout images through the neck are obtained, followed by a limited helical acquisition, extending to the level above and below the facet joints to be denervated (kVp 120, tube current 20–100 mA average 30 mA, 1 mm contiguous slice thickness). As each facet joint is supplied by the medial branch from the level above and below, a minimum of two nerves are denervated per procedure.
The pre-procedure images are reviewed on soft tissue windows to assess the location of the jugular vein, carotid and vertebral arteries. Prior CT or MRI imaging is also reviewed for any aberrant vasculature, if available. A skin entry site along the lateral neck that will allow access to the target location, whilst avoiding these structures is chosen. Typically, the entry point and needle trajectory will be posterior to the carotid sheath structures, with the needle angled slightly downwards.
The entry site is marked on the patient’s skin. Standard aseptic technique is utilized and 2–3 mL of 1% lignocaine is administered subcutaneously at each level. A 10-cm 20-gauge straight radiofrequency cannula with 10 mm tip exposure (Cosman CC101020-P) is advanced to the landmark using intermittent CT fluoroscopy. The exposed tip is carefully positioned to ensure lesioning of the medial branch both lateral and dorsolateral to the pillar (Figure 2A).
Once satisfactory tip position is confirmed, the stylet is removed from the cannula and a length matched sterile reusable denervation probe is inserted and attached to the RF generator (Cosman G4 v2.1.0). A single thermal continuous RF lesion is generated at a temperature of 90 degrees Celsius for 90 seconds. The denervation probe is then removed and 1.5 mL of 0.5% Bupivacaine local anaesthetic is injected via the RF cannula prior to removal.
Once all ipsilateral levels have been denervated, the process can be repeated for any contralateral levels. The patient is observed for a minimum of 2 hours post-procedure prior to discharge.
Discussion
At our institution, CT guidance is utilized for the majority of spinal procedures and is a technique familiar to both general and interventional radiologists. CT guided intervention allows rapid and accurate needle positioning. Direct visualisation of the needle tip and its relation to regional soft tissue and neurovascular structures provides theoretical safety benefits, potentially avoiding inadvertent lesioning of the spinal nerve or spinal cord, which have been reported as rare complications (9).
Most traditional fluoroscopic techniques utilize a posterior approach with the patient prone. For CT guided intervention, the supine position is preferred by anaesthetists at our institution due to safer airway management and control. We have adapted our technique to the supine position using a lateral approach, similar to the approach utilised for CT guided cervical nerve root injections (10). We position the needle at the dorsolateral aspect of the pillar where it remains parallel to the medial branch, an important consideration as heat generation is predominantly sideways and parallel to the needle shaft (11). We utilise a 10 mm exposed tip which enables lesioning of the lateral and dorsolateral medial branch (Figure 2A).
We do not routinely perform motor or sensory nerve stimulation prior to lesion generation. We are aware that many practitioners utilise this as an adjunct to fluoroscopic imaging to ensure adequate distance from major neurological elements prior to nerve coagulation, and this could be adopted depending on proceduralist preference.
At our institution we generate a single lesion at each level. We recognize that many practitioners advocate generating multiple RF lesions at each level to allow for slight inter-individual variation in nerve course. The CT guided technique we have described could be modified to suit individual practices.
To date, we have performed cervical medial branch ablations in over 90 patients using the technique described, with 70% of patients receiving clinical benefit, which is similar to published results for the traditional fluoroscopic technique (8). Twenty percent of patients have required repeat ablations at between 9–24 months due to neural regrowth. There have been no major complications, with a small percentage (10%) of patients describing transient cutaneous numbness, whilst one patient had ongoing sensory cutaneous changes which have not caused significant clinical concern to the patient.
We recognise our modified technique may have limitations. Compared to the fluoroscopic technique as described by Lord (6), we lesion the medial branch in a more distal location where there is greater potential for variability of nerve course. Given our needle tip position differs slightly from traditional fluoroscopic techniques, extrapolation of efficacy data from previous published studies may not be reliable.
Our technique utilizes intermittent CT fluoroscopy and low mA to limit radiation exposure. We have not quantified the level of radiation dose, however it has been demonstrated that CT guided procedures can be performed with acceptably low radiation doses to both patient and radiologist (12).
Conclusions
CT guided cervical medial branch denervation via a lateral approach is a viable alternative to traditional fluoroscopic techniques.
Acknowledgements
This work was supported by Dr. Jones and Partners Medical Imaging, St Andrews Hospital, Adelaide, SA 5000, Australia.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was performed following approval from the St Andrews Hospital Human Research Ethics Committee. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 [5].
References
- Yin W, Bogduk N. The nature of neck pain in a private pain clinic in the United States. Pain Med 2008;9:196-203. [Crossref] [PubMed]
- Manchikanti L, Manchikanti KN, Pampati V, et al. The prevalence of facet-joint-related chronic neck pain in postsurgical and nonpostsurgical patients: a comparative evaluation. Pain Pract 2008;8:5-10. [Crossref] [PubMed]
- Barnsley L, Lord SM, Wallis BJ, et al. The prevalence of chronic cervical zygapophyseal joint pain after whiplash. Spine (Phila Pa 1976) 1995;20:20-5; discussion 26. [Crossref] [PubMed]
- Lord SM, Barnsley L, Wallis BJ, et al. Chronic cervical zygapophysial joint pain after whiplash. A placebo-controlled prevalence study. Spine (Phila Pa 1976) 1996;21:1737-44; discussion 1744-5.
- Klessinger S. Radiofrequency neurotomy for the treatment of therapy-resistant neck pain after ventral cervical operations. Pain Med 2010;11:1504-10. [Crossref] [PubMed]
- Lord SM, Barnsley L, Wallis BJ, et al. Percutaneous radio-frequency neurotomy for chronic cervical zygapophyseal-joint pain. N Engl J Med 1996;335:1721-6. [Crossref] [PubMed]
- McDonald GJ, Lord SM, Bogduk N. Long-term follow-up of patients treated with cervical radiofrequency neurotomy for chronic neck pain. Neurosurgery 1999;45:61-7; discussion 67-8. [PubMed]
- MacVicar J, Borowczyk JM, MacVicar AM, et al. Cervical medial branch radiofrequency neurotomy in New Zealand. Pain Med 2012;13:647-54. [Crossref] [PubMed]
- Falco FJ, Manchikanti L, Datta S, et al. Systematic review of the therapeutic effectiveness of cervical facet joint interventions: an update. Pain Physician 2012;15:E839-68. [PubMed]
- Wagner AL. CT fluoroscopic-guided cervical nerve root blocks. AJNR Am J Neuroradiol 2005;26:43-4. [PubMed]
- Centeno CJ, Thacker J, Elkins W. Radiofrequency lesioning of the cervical medial branches. Techniques in Regional Anaesthesia and Pain Management 2004;8:10-6. [Crossref]
- Wagner AL. Selective lumbar nerve root blocks with CT fluoroscopic guidance: technique, results, procedure time, and radiation dose. AJNR Am J Neuroradiol 2004;25:1592-4. [PubMed]


