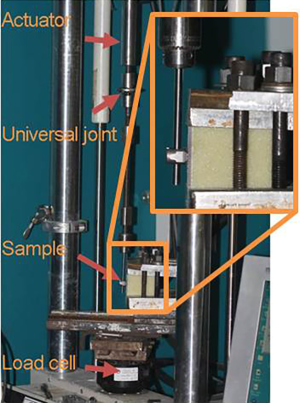
The contribution of the cortical shell to pedicle screw fixation
Introduction
Pedicle screw and rod systems are commonplace in spinal fusion surgery. Pedicle screws require initial rigid fixation and longevity to stabilise the spine while the fusion mass forms. This procedure is broadly successful with low rates of screw loosening for standard screws (1-3) and increased rates for dynamic systems (4-7). Screw loosening is a concern, with radiolucency around screws detrimental to fixation (8) and often warranting revision surgery (9).
Pedicle screws implanted using traditional techniques produce a characteristic butterfly shaped defect following Caudo-cephalad loading (10). Achieving bicortical screw fixation provides anchoring of the screw in the far cortex to improve stability (11), but not without risks to structures near the exiting screw (12-14). A technique known as “hubbing”, whereby the cortical shell is removed immediately adjacent to the pedicle screw insertion hole, allows deeper insertion of the screw. This is intended to reduce the lever arm thereby reducing load and ultimately motion at the tip of the screw to achieve fixation similar to bicortical placement (15). Paik et al. (15) investigated early loading (toggling) on specimens with and without hubbing and found that hubbed and toggled specimens had lower pullout strength as compared to those that were only toggled, osteoporotic specimens also showed lower pullout than their normal BMD counterparts. A directly comparative assessment of pullout without toggling is still lacking.
It is well known that BMD is an important factor affecting pullout loads (16-18). However, it is unknown if an alteration in BMD can predispose bone to toggling damage following hubbing. Screw loosening has been shown to be more common in osteoporotic patients (19). The majority of screw stability is attained through fixation with cortical bone which suggests that hubbing may pose a greater risk to these patients (20). This study used a reproducible biomechanical model for evaluating toggling to investigate individual and interaction effects of toggling, hubbing, and bone quality.
Methods
Synthetic bone blocks (60 mm × 40 mm × 62 mm) with cancellous bulk and a simulated cortical shell of 2 mm thick short fiber filled epoxy layer (Sawbones, Vashaw, WA) were used in this study. The cortical shell was predrilled with a three fluted 3.2 mm drill bit (Surgibit, Orthopedic Innovations, Collaroy, NSW, Australia) followed by a 5 mm tap and the placement of the screws. G5 multiaxial pedicle screws (diameter × long: 6.5 mm × 45 mm) (KH Medical Pty Ltd, Sydney, Australia) were implanted into the bone block followed by the G5 Rod 6.5 mm and G5 Cap using the manufacturers instrumentation and according to instructions for use.
Three key variables evaluated in this study were density of the simulated bone, toggling before pullout testing, and the presence or absence of the surrounding cortex (hubbing). This resulted in eight groups with n=8 samples per group based on pre-hoc power calculations. The cancellous core was normal density, 10# cellular foam (10 lb/ft3) or low density, 20# cellular foam (20 lb/ft3). Dynamic toggle testing was performed in reverse cycle tension-compression at 5 Hz in air at room temperature at ±0.5 mm for 10,000 cycles on a calibrated servo hydraulic mechanical testing machine (MTS Systems Corporation, Eden Prairie, MN). Samples were rigidly mounted to the load cell and the actuator incorporated a universal joint and chuck to accommodate alignment (Figure 1). Hubbed samples had the cortex removed to a radius of 5 mm hole using a high-speed handpiece (Midas Rex, Medtronic, Fort Worth, TX, USA) with a matchstick burr (M-8/9MH30). Each sample was tested in uniaxial pullout (tension) at 5 mm per minute after the toggling loading. Maximum load, stiffness of the linear region, and energy to peak load were determined. Results were analyzed with a multivariate ANOVA. Post hoc t-tests with a significance level of 0.05 were performed to identify differences between individual groups where appropriate using SPSS.
Results
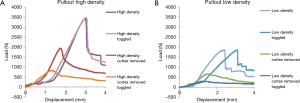
All samples completed the toggling without evidence of failure. Qualitatively, it was evident that the load versus displacement pull-out curves varied between groups. Samples with the cortex typically exhibited a steep climb and a sharp drop off during failure while groups with the cortex removed showed a more gradual failure (Figure 2).
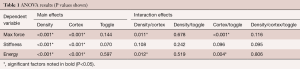
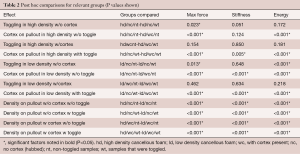
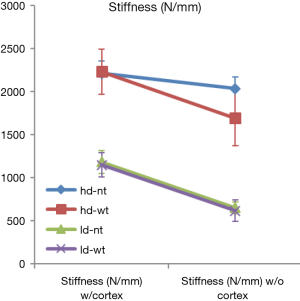
The ANOVA results indicated main effects for Density and Cortex (hubbing) were present for maximum force and energy along with interaction effects (Tables 1,2). Stiffness had no interaction effects and has a more straightforward interpretation. Stiffness was decreased by the removal of the cortex for all but the non-toggled high-density group with the cortex in place (Figure 3). While the effect of decreasing cancellous density on stiffness was clearly negative, toggling did not have an effect on this property.
Full table
Full table
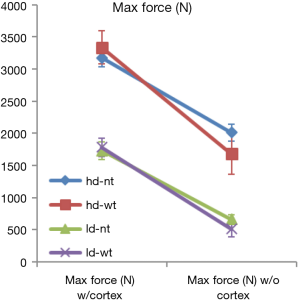
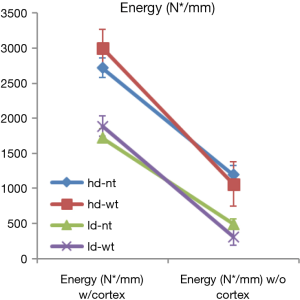
Maximum force and energy to failure both showed interaction effects of density/cortex and cortex/toggle. The effects of cortex removal are apparent due to the downward sloping lines in all samples. The interaction effects of cortex/toggle appear as crossed lines within the same density, showing additional loss of pullout load and energy of failure (Figures 4,5) when hubbing and toggling are combined. The interaction effects of density/cortex are slightly more challenging to see plainly but the higher density Sawbones pullout properties were reduced to a greater extent than the low-density samples.
Discussion
Bone density and strength vary based on patent health and underlying pathology. Osteopenic and osteoporotic bone is often encountered when performing spine surgery, influencing not only fracture risk also hardware fixation (9,21). Polyurethane foam is a well-established bone surrogate for biomechanical testing (22,23) and although there are some limitations, simulation of normal and osteoporotic bone can be achieved with 0.32 g/cc3 (20 lb/ft3) and 0.16 g/cc3 (10 lb/ft3) foam respectively (24). This study used two densities of simulated bone in a pedicle screw pullout model with and without toggling and hubbing. These factors were chosen to simulate clinically relevant variables.
Several studies have investigated factors influencing pedicle screw stability, such as trajectory (25,26), hole preparation (27), reuse of pilot holes (28) and screw back out (29). The removal of the cortex immediately surrounding the pedicle screw simulates a variation on screw placement known as hubbing. Concerns regarding this technique include reduced pullout loads (15,30). In our study, removal of the cortex not only decreased the pull-out loads alone but further weakened the fixation of the screws when subjected to toggling. Toggling simulates Caudo-cephalad loading occurring during activities of daily living (10). Toggling in the presence of the cortical shell had no effect. However, once the cortical shell is removed, damage to the weaker cancellous bone accumulates and further weakens the fixation. This demonstrates that this combination of treatments is worse than either alone. Weaker cancellous bone produced lower pull-out strength, as expected, and this difference was considerable for the group with the removed cortical layer, decreasing maximum pull-out load to less than one third that of the normal density samples. This value was similar for both low and high-density groups but due to the lower starting point of the low density group the proportional reduction and final properties were considerably greater. This suggests that screw fixation is more vulnerable in the presence of cortical damage in weaker, or potentially osteopenic bone.
Comparison to existing literature
Screw pull-out studies using human cadaveric tissues report a wide range of values from 102 to 1,741 N (15,26,28,31-34). Similarly, screw pull-out with the calf model reports values from 747 to 7,300 N, depending on treatment (30,35). Polyurethane blocks have been reported in the literature and offer a wealth of data to compare against. High density blocks demonstrate pullout loads of 2,908 N (27) for 7.5 mm screws, 2,115–2,227 N (29) and 2,133 N (36) for 7.0 mm screws, 2,652 N (37) for 6.5 mm screws and 1,420–2,176 with a 6 mm (38) screws. The comparable condition in the current study generated 2,011 N with 6.5 mm screws. Pfeiffer and Abernathie (27) have evaluated 6.25, 6.5, 6.75 and 7.5 mm sizes, the results of which are more fully compared in Figure 6. These values are higher than the majority of cadaveric pullout studies and are more closely comparable to the calf or porcine spine results (37,39).
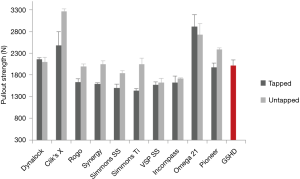
A few studies have investigated lower density foam as well and are comparable to the low-density group in the present study. Low density with 6.5 mm generated 688 N (36). With 6 and 6.2 mm screws, it is 307–727 N (38). This compares well with the current loads of 661 N. Kim et al. (40) have extensively investigated the effects specific design factors (inner and outer diameter shape and thread shape and tested them in 3 density ranges (5, 15 and 20 lb/ft3). These results are compared in Figure 7. Design variations included diameters (6.5 mm as in the current study) that were cylindrical or conical and threads including V, buttress, and square shapes. Results showed that V-shaped threads had the maximum pullout strength, regardless of bone density, diameter, shape and type of threads. The screw tested in the current study had a conical outer diameter and a two-stage outer diameter for cortical/cancellous bone and buttress shaped threads. When comparing these results, we see that the current screw achieves pullout loads in 10 lb/ft3 bone surrogate that are reported in 15 lb/ft3 foam. The current study evaluated pedicle screw pullout characteristics in simulated bone in the presence of a cortex. This feature was structurally significant and added approximately 1,100 N to the pullout load.

Caudo-cephalad loading in an in vitro setting (10) is well established and can be used as a preconditioning step prior to pullout or can itself be an endpoint for loosening (41-46). Toggling generally decreases the stability of the screw/bone construct (11,32), however, this is not always the case. Lotz et al. (47) reported pullout loads of 809±467 N without cyclic loading and 801±265 N following cyclic loading. Likewise, there are some reports that do not agree as closely with the current study. Patel et al. (48) evaluated the effect of toggling with 4.7 and 6.5 mm pedicle screws in 20 and 10 lb/ft3 foam bone. They found no difference with toggling despite having a more aggressive toggling protocol (±1 vs. ±0.5 mm). Also of note, the loads were much lower than the current study: 1,110 N (6.7 mm screw) vs. 2,011 N (6.5 mm screw) for high density non-toggled pullout. There are several possible reasons for this, including a screw length of 30 vs. 45 mm. Stiffness of the testing setup is very important for a displacement controlled toggle and, although the test setup shown in the paper appeared sound, the use of test block anchor screws rather than a plate to restrain the block may have allowed the anchor screws to toggle as well as the pedicle screw, minimizing the motion at the pedicle screw/foam interface. Likewise, Mehmanparast et al. (49) performed a comparable study and found that toggling decreased pullout loads significantly for 20 lb/ft3 but not 10 or 30 lb/ft3 foam while stiffness was affected for all. Values were again lower but this may have been due to the smaller, 5mm screw. Interestingly, both of these studies report a non-significant increase in pullout strength for the low-density foam following toggling.
Investigations into hubbing are less common. A key publication on the topic comes from Paik et al. (15) who examined pullout following hubbing and toggling for cadaveric samples that were classified as normal or osteoporotic. A significant drop in pullout load from 772 to 418 N (normal) and from 414 to 243 N (osteoporotic) was found. This represents a drop of 41% and 46% of the intact values. On a comparative basis, our results demonstrate a decrease of the intact value of 47% for the high-density group and a more substantial 70% drop in the low-density group. The trend was similar but the greater magnitude of the differences may be due to the relative differences between the foam densities being greater that found in cadaveric cancellous bone. Unfortunately, all samples were toggled and the effects of toggling, hubbing and bone density could not be individually assessed. Lill et al. (35) tested screws in calf spines and removed the effect of thicker cortex, as compared to humans, by over-drilling the dorsal cortex to 8 mm diameter and a depth of 2 mm. Although this study did not state the intention to evaluate hubbing, the procedure was very similar. The loads in this study were high (1.8–7.3k N) and unexpectedly, suggest that the bone became stronger following toggling.
Finally, Kang et al. (30) also tested the calf spine and found significantly lower pullout following toggling, also noting dorsal cortex deformation and micro fractures in cases after hubbing plus toggling.
The use of synthetic Sawbone bone surrogates has limitations. The properties of maximum pullout load and stiffness are much higher than that reported for human cadaveric bone. This is well documented and considering the reduced variability, this material is useful for comparative tests. The addition of a cortical layer is significant and demonstrates a more realistic model of load sharing. The addition of simulated pedicle geometry with cortical and cancellous layers would further aid in improving the accuracy of the model.
Conclusions
This study has presented an evolution of a well-established screw pullout/toggle testing model. The cortex plays a considerable role in the protection of underlying cancellous bone as well as contributing to initial pullout strength. Removal of the cortex not only decreased the pullout loads alone but further weakened the fixation of the screws when subjected to toggling. Toggling in the presence of the cortical shell had no effect. However, once the cortical shell is removed damage to the weaker cancellous bone accumulates and further weakens the fixation. Our idealised in vitro results suggest the existence of a detrimental interactive effect when both toggling and hubbing are applied to pedicle screws implanted in weaker, osteoporotic bone.
Acknowledgements
This work was supported by KH Medical Pty Ltd., Sydney, Australia who provided all implants, instruments for implantation and consumables.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Esses SI, Sachs BL, Dreyzin V. Complications Associated with the Technique of Pedicle Screw Fixation A Selected Survey of ABS Members. Spine 1993;18:2231-8. [Crossref] [PubMed]
- Suk SI, Kim WJ, Lee SM, et al. Thoracic pedicle screw fixation in spinal deformities: are they really safe? Spine 2001;26:2049-57. [Crossref] [PubMed]
- Faraj AA, Webb J. Early complications of spinal pedicle screw. Eur Spine J 1997;6:324-6. [Crossref] [PubMed]
- Bothmann M, Kast E, Boldt GJ, et al. Dynesys fixation for lumbar spine degeneration. Neurosurg Rev 2008;31:189-96. [Crossref] [PubMed]
- Stoll TM, Dubois G, Schwarzenbach O. The dynamic neutralization system for the spine: a multi-center study of a novel non-fusion system. Eur Spine J 2002;11:S170-8. [PubMed]
- Korovessis P, Papazisis Z, Koureas G, et al. Rigid, semirigid versus dynamic instrumentation for degenerative lumbar spinal stenosis: a correlative radiological and clinical analysis of short-term results. Spine 2004;29:735-42. [Crossref] [PubMed]
- Wu J-C, Huang W-C, Tsai H-W, et al. Pedicle screw loosening in dynamic stabilization: incidence, risk, and outcome in 126 patients. Neurosurg Focus 2011;31:E9. [Crossref] [PubMed]
- Sandén B, Olerud C, Petren-Mallmin M, et al. The significance of radiolucent zones surrounding pedicle screws definition of screw loosening in spinal instrumentation. J Bone Joint Surg Br 2004;86:457-61. [Crossref]
- Ponnusamy KE, Iyer S, Gupta G, et al. Instrumentation of the osteoporotic spine: biomechanical and clinical considerations. Spine J 2011;11:54-63. [Crossref] [PubMed]
- Law M, Tencer AF, Anderson PA. Caudo-cephalad loading of pedicle screws: mechanisms of loosening and methods of augmentation. Spine 1993;18:2438-43. [Crossref] [PubMed]
- Zindrick MR, Wiltse LL, Widell EH, et al. A biomechanical study of intrapeduncular screw fixation in the lumbosacral spine. Clin Orthop Relat Res 1986.99-112. [PubMed]
- Licht NJ, Rowe DE, Ross LM. Pitfalls of Pedicle Screw Fixation in the Sacrum: A Cadaver Model. Spine 1992;17:892-6. [Crossref] [PubMed]
- Esses SI , Bostford D, Huler R, et al. Surgical Anatomy of the Sacrum: A Guide for Rational Screw Fixation. Spine 1991;16:S283-8. [Crossref] [PubMed]
- Ergur I, Akcali O, Kiray A, et al. Neurovascular risks of sacral screws with bicortical purchase: an anatomical study. Eur Spine J 2007;16:1519-23. [Crossref] [PubMed]
- Paik H, Dmitriev AE, Lehman RA Jr, et al. The biomechanical effect of pedicle screw hubbing on pullout resistance in the thoracic spine. Spine J 2012;12:417-24. [Crossref] [PubMed]
- Halvorson TL, Kelley LA, Thomas KA, et al. Effects of bone mineral density on pedicle screw fixation. Spine 1994;19:2415-20. [Crossref] [PubMed]
- Okuyama K, Abe E, Suzuki T, et al. Can insertional torque predict screw loosening and related failures?: An in vivo study of pedicle screw fixation augmenting posterior lumbar interbody fusion. Spine 2000;25:858-64. [Crossref] [PubMed]
- Okuyama K, Sato K, Abe E, et al. Stability of Transpedicle Screwing for the Osteoporotic Spine: An In Vitro Study of the Mechanical Stability. Spine 1993;18:2240-5. [Crossref] [PubMed]
- Wu ZX, Gong FT, Liu L, et al. A comparative study on screw loosening in osteoporotic lumbar spine fusion between expandable and conventional pedicle screws. Arch Orthop Trauma Surg 2012;132:471-6. [Crossref] [PubMed]
- Hirano T, Hasegawa K, Takahashi HE, et al. Structural characteristics of the pedicle and its role in screw stability. Spine 1997;22:2504-9. [Crossref] [PubMed]
- Lehman RA, Kang DG, Wagner SC. Management of Osteoporosis in Spine Surgery. J Am Acad Orthop Surg 2015;23:253-63. [Crossref] [PubMed]
- ASTM. ASTM F1839, standard specification for rigid polyurethane foam for use as a standard material for testing orthopaedic devices and instruments. American Society for Testing and Materials. West Conshohocken: ASTM, 2008.
- Calvert KL, Trumble KP, Webster TJ, et al. Characterization of commercial rigid polyurethane foams used as bone analogs for implant testing. J Mater Sci Mater Med 2010;21:1453-61. [Crossref] [PubMed]
- Patel PS, Shepherd DE, Hukins DW. Compressive properties of commercially available polyurethane foams as mechanical models for osteoporotic human cancellous bone. BMC Musculoskelet Disord 2008;9:137. [Crossref] [PubMed]
- Farshad M, Farshad-Amacker NA, Bachmann E, et al. Biomechanical comparison of sagittal-parallel versus non-parallel pedicle screw placement. Acta Neurochir (Wien) 2014;156:2147-51. [Crossref] [PubMed]
- Baluch DA, Patel AA, Lullo B, et al. Effect of Physiological Loads on Cortical and Traditional Pedicle Screw Fixation. Spine 2014;39:E1297-302. [Crossref] [PubMed]
- Pfeiffer FM, Abernathie DL, Smith DE. A comparison of pullout strength for pedicle screws of different designs: a study using tapped and untapped pilot holes. Spine 2006;31:E867-70. [Crossref] [PubMed]
- Kang DG, Lehman RAJ, Wagner SC, et al. Pedicle Screw Reinsertion Using Previous Pilot Hole and Trajectory Does Not Reduce Fixation Strength. Spine 2014;39:1640-7. [Crossref] [PubMed]
- Amaritsakul Y, Chao CK, Lin J. Comparison study of the pullout strength of conventional spinal pedicle screws and a novel design in full and backed-out insertions using mechanical tests. Proc Inst Mech Eng H 2014;228:250-7. [Crossref] [PubMed]
- Kang DG, Lehman RA Jr, Bevevino AJ, et al. Pedicle Screw “Hubbing” in the Immature Thoracic Spine: A Biomechanical and Micro-Computed Tomography Evaluation. J Pediatr Orthop 2014;34:703-9. [Crossref] [PubMed]
- Chao KH, Lai YS, Chen WC, et al. Biomechanical analysis of different types of pedicle screw augmentation: A cadaveric and synthetic bone sample study of instrumented vertebral specimens. Med Eng Phys 2013;35:1506-12. [Crossref] [PubMed]
- Karami KJ, Buckenmeyer LE, Kiapour AM, et al. Biomechanical Evaluation of the Pedicle Screw Insertion Depth Effect on Screw Stability Under Cyclic Loading and Subsequent Pullout. J Spinal Disord Tech 2015;28:E133-9. [Crossref] [PubMed]
- Cook SD, Salkeld SL, Stanley T, et al. Biomechanical study of pedicle screw fixation in severely osteoporotic bone. Spine J 2004;4:402-8. [Crossref] [PubMed]
- Vishnubhotla S, McGarry WB, Mahar AT, et al. A titanium expandable pedicle screw improves initial pullout strength as compared with standard pedicle screws. Spine J 2011;11:777-81. [Crossref] [PubMed]
- Lill CA, Schlegel U, Wahl D, et al. Comparison of the in vitro holding strengths of conical and cylindrical pedicle screws in a fully inserted setting and backed out 180. J Spinal Disord 2000;13:259-66. [Crossref] [PubMed]
- Hashemi A, Bednar D, Ziada S. Pullout strength of pedicle screws augmented with particulate calcium phosphate: an experimental study. Spine J 2009;9:404-10. [Crossref] [PubMed]
- Inceoğlu S , Ehlert M, Akbay A, et al. Axial cyclic behavior of the bone–screw interface. Med Eng Phys 2006;28:888-93. [Crossref] [PubMed]
- Krenn MH, Piotrowski WP, Penzkofer R, et al. Influence of thread design on pedicle screw fixation. Laboratory investigation. J Neurosurg Spine 2008;9:90-5. [Crossref] [PubMed]
- Abshire BB, McLain RF, Valdevit A, et al. Characteristics of pullout failure in conical and cylindrical pedicle screws after full insertion and back-out. Spine J 2001;1:408-14. [Crossref] [PubMed]
- Kim YY, Choi WS, Rhyu KW. Assessment of pedicle screw pullout strength based on various screw designs and bone densities-an ex vivo biomechanical study. Spine J 2012;12:164-8. [Crossref] [PubMed]
- Waits C, Burton D, McIff T. Cement augmentation of pedicle screw fixation using novel cannulated cement insertion device. Spine 2009;34:E478-83. [Crossref] [PubMed]
- Kiner DW, Wybo CD, Sterba W, et al. Biomechanical analysis of different techniques in revision spinal instrumentation: larger diameter screws versus cement augmentation. Spine 2008;33:2618-22. [Crossref] [PubMed]
- Hasegawa K, Takahashi HE, Uchiyama S, et al. An experimental study of a combination method using a pedicle screw and laminar hook for the osteoporotic spine. Spine 1997;22:958-62. [Crossref] [PubMed]
- Inceoglu S, Ferrara L, McLain RF. Pedicle screw fixation strength: pullout versus insertional torque. Spine J 2004;4:513-8. [Crossref] [PubMed]
- Sterba W, Kim D-G, Fyhrie DP, et al. Biomechanical analysis of differing pedicle screw insertion angles. Clin Biomech (Bristol, Avon) 2007;22:385-91. [Crossref] [PubMed]
- Hoppe S, Loosli Y, Baumgartner D, et al. Influence of screw augmentation in posterior dynamic and rigid stabilization systems in osteoporotic lumbar vertebrae: a biomechanical cadaveric study. Spine 2014;39:E384-9 . [Crossref] [PubMed]
- Lotz JC, Hu SS, Chiu DF, et al. Carbonated apatite cement augmentation of pedicle screw fixation in the lumbar spine. Spine 1997;22:2716-23. [Crossref] [PubMed]
- Patel P, Hukins D, Shepherd D. The Effect of “Toggling” on the Pullout Strength of Bone Screws in Normal and Osteoporotic Bone Models. Open Mechanical Engineering Journal 2013;7:35-9. [Crossref]
- Mehmanparast H, Mac-Thiong JM, Petit Y. Biomechanical evaluation of pedicle screw loosening mechanism using synthetic bone surrogate of various densities. Engineering in Medicine and Biology Society (EMBC), 2014 36th Annual International Conference of the IEEE. 2014, IEEE.