Interlaminar endoscopic lateral recess decompression—surgical technique and early clinical results
Introduction
Degenerative changes of discs, facet joints and ligamentum flavum may lead to narrowing of the spinal canal (stenosis) and neurological symptoms once neurovascular structures are entrapped (1). The diagnosis of lumbar spinal stenosis is common among patients older than 65 years old (2). The Framingham population study found that 19–47% of Americans older than 60 years of age display radiographic evidence of spinal stenosis depending on criteria used. Moreover, 13–14% of patients with low back problems who see a specialist and 3–4% of patients who see a general physician are diagnosed with lumbar spinal stenosis (3). Accordingly, spinal stenosis is the most common indication for spine surgery in patients older than 65 years (4). Anatomically, the spinal canal has been divided in two main regions, central and nerve root canal. Stenosis can occur in either or both regions. The nerve root canal has been divided to three zones: lateral recess, foraminal region, and extra-foraminal region (5). The current report focuses on the lateral recess which constitutes the entrance zone for lumbar nerve roots entering the nerve root canal. The lateral recess is localized underneath the superior articular process and is confined by anterior and posterior walls. The anterior wall is formed by the annulus of the disc and the posterior wall by the facet joint (5) (Figure 1). The most common pathologies leading to lateral recess stenosis are hypertrophic facet joint osteoarthritis, bulging of the disc annulus or posterior endplate osteophytes (5-7). Diagnosis of symptomatic lateral recess stenosis is controversial. Neither optimal imaging modality nor radiographic criteria have been established for diagnosis of lateral recess stenosis. The North American Spine Society suggests MRI as the best noninvasive imaging modality to evaluate the spinal canal anatomy in patients with radicular symptoms (1). However, the sensitivity of current MRI criteria to detect lateral recess stenosis has been suggested to be only approximately 60% (8). Conventional myelography has been proposed to have a higher sensitivity to detect lateral recess stenosis (8). Moreover, a great variety of radiographic criteria such as lateral recess height, depth and angle have been proposed (8-11).
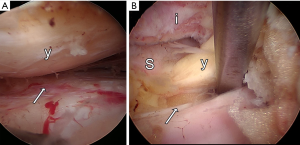
Symptomatic lateral recess stenosis is typically first treated with conservative management includes NSAIDs, physiotherapy, spinal injections, lifestyle modification, and multidisciplinary rehabilitation (12). Surgery is recommended for patients who fail to response to non-surgical treatments (13). Surgical procedures aim to decompress the nerve root emerging from the thecal sac along its course in the lateral recess. A multitude of surgical procedures for surgical decompression of symptomatic lateral recess stenosis have been described ranging from standard open laminectomies to minimally invasive decompressive techniques (14-22). In the current study we describe the surgical technique of endoscopic interlaminar lateral recess decompression in a small cohort of patients with unilateral symptomatic lateral recess stenosis with well-defined radiographic criteria.
Methods
Patient cohort
Our prospective database of 146 endoscopic spine procedures performed at the University of Washington between September 2014 and May 2016 was screened for patients who had undergone endoscopic medial facetectomy for unilateral single level symptomatic lateral recess stenosis by a single surgeon (Christoph P. Hofstetter). All patients had unilateral radicular symptoms from impingement of the traversing nerve root within the lateral recess. Patients included in the current cohort had failed conservative therapy including at least 6 weeks of physiotherapy, NSAIDs, nerve blockade or epidural steroid injections. Patients were excluded if they had symptomatic central canal stenosis, symptomatic contralateral lateral recess stenosis, dynamic instability (more than 3 mm motion on flexion/extension X-rays) or a sequestered disc fragment. We recorded patient demographics, preoperative imaging, operative details, clinical outcomes, and complications. Outcomes were measured using VAS and ODI scores (23) at 2 weeks, 3 months and at last follow-up. For the ODI, 12 points were regarded as the minimally clinically important difference (MCID) (24-26) and for the VAS the MCID was 3 points (24,27,28).
Preoperative imaging
All patients included in the current report underwent preoperative flexion and extension X-rays of the lumbar spine to rule out dynamic instability. Dynamic instability was defined as more than 3 mm translational movement in on anteroposterior (AP) direction (29). Preoperative MRI studies of the lumbar spine were reviewed independently by Zeinab Birjandian and Christoph P. Hofstetter and values averaged. T2-weighted axial images parallel to the axis of the index level intervertebral disc were analyzed. Lateral recess angle and height were measured to quantify lateral recess stenosis (9-11,30,31). The lateral recess angle was measured between tangents of the disc annulus and the facet joint centered in middle of the traversing nerve root. The lateral recess height was measured along a sagittal plane at the medial margin of the traversing nerve root. If the nerve root was not visible due to severe compression the medial margin and the middle of the nerve root were determined at the adjacent rostral and caudal images and the mediolateral nerve root location was averaged (Figure 2). We also measured the area of the nerve root at the site of maximum compression as well as at the midpedicular level of the caudal segment. Measurements were carried out on a PACS workstation (Centricity PACS, GE Healthcare).
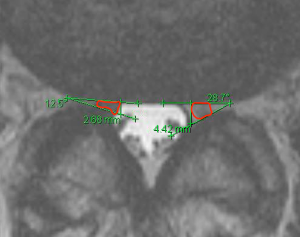
Surgical technique
Set-up and approach
The patient undergoes general endotracheal anesthesia and is positioned prone on a Jackson table with a Wilson frame. Attention is given to maximize kyphosis of the patient, this may be achieved by raising the Wilson frame or adding rolls underneath the anterior superior iliac spine. Once the patient is prepped and draped, an AP X-ray is obtained to determine the appropriate level and the optimal craniocaudal angle of the surgical corridor. Typically, tilting the C-arm 10–15 degrees caudally from an initial endplate view of the caudal vertebral body allows to minimize removal of the inferior articular process. Utilizing the determined craniocaudal tilt, the skin incision is marked where the inferior margin of the lamina intersects with the middle of the disc space (Figure 3). A stab incision is made through skin and thoracolumbar fascia using an 11-blade. The trocar is advanced through the incision toward the inferior margin of the lamina. The caudal margin of the lamina is palpated with the trocar. Excessive movement of the trocar should be avoided to minimize muscular bleeding. Typically, one more confirmatory AP X-ray is obtained and the c-arm is moved out of the operative field. Then, the working cannula with the bevel facing medially is introduced over the trocar. The working cannula needs to be advanced until the lamina is palapated, insufficient advancement will lead in incomplete retraction of the paraspinal muscles. The trocar is then removed and the endoscope is introduced. The Trigger Flex bipolar electrode and micro punches are utilized to define the inferior margin of the lamina and the medial aspect of the facet joint.
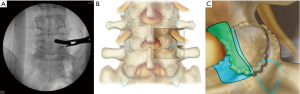
Decompression
A high-speed diamond drill is used to resect the caudal portion of the rostral lamina and the medial aspect of the facet joint (Figures 3 and 4A). At this point, the yellow ligament is identified and dissected along its fibers under using the open micro punches (Figure 4B). Yellow ligament is resected piecemeal using micro punches and kerrison rongeurs. At this point the thecal sac is visualized and the lateral margin of the traversing nerve root is identified (Figure 4C). The high speed diamond drill is used to undercut the facet joint until the lateral margin of the traversing nerve root is decompressed. Given the 25 off axis optics of the endoscope, rotation of the endoscope helps with direct visualization of the nerve root and to undercut the facet joint. At this point the superior articular process is identified. It is resected along the lateral margin of the traversing nerve root together with the most rostral part of the rostral portion of the next level lamina (Figure 4D). Gentle lateral pressure onto the inferior articular process facilitates undercutting of the joint. Once the traversing nerve is visualized it is mobilized using the blunt dissector. Adhesions that cannot be freed by blunt dissection are sharply dissected using the scissors and/or Trigger Flex. The annulus and endplates anterior to the traversing nerve root are visualized. Once the nerve is mobilized a small side biting drill is used to obtain a smooth bony edge lateral to the nerve root. Direct visualization of the traversing nerve root should be obtained from the tip of the superior articulating facet to the midpedicular line of the caudal pedicle, which may be verified using fluoroscopy.
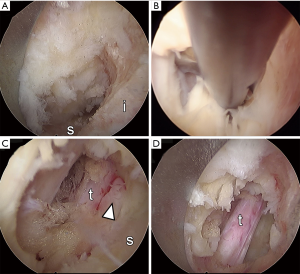
Closure
Once hemostasis is achieved the endoscope and working cannula are removed. Closure is carried out in a layered fashion, with 0 Vicryls for the subcutaneous tissue, followed by a subcuticular 4-0 biosyn for the skin. Steri strips are placed to approximate the wound edges and the wound is covered with primapore.
Statistical analysis
Continuous variables are shown as means ± standard error of the mean (SEM). Lateral recess angles and height between symptomatic and non-symptomatic side were compared using the nonparametric Wilcoxon Signed Rank test. Repeated measurements including VAS and ODI were compared using a paired-sample T-test. Statistical calculations were carried out using SPSS 24 for Mac.
Results
Patients
The current study includes ten patients with unilateral symptomatic lateral recess stenosis. The patient group consisted of four men and six women with an average age of 58.4 (range, 36–72) years. The average duration of symptoms was 16.2±4.6 months prior to surgery. All patients had radicular pain symptoms consistent with the nerve root involved. Focal lower extremity weakness was observed in 70% (7 out of 10). None of the patients had bladder or bowel dysfunction.
Radiographic diagnostic criteria
The diagnosis was based on a combination of unilateral radicular leg pain with corresponding ipsilateral lateral recess stenosis. Measurements of the lateral recess angle at the symptomatic level (L3/4 for L4 radiculopathy, L4/5 for L5 radiculopathy, and L5/S1 for S1 radiculopathy) revealed that the lateral recess angle was significantly smaller (19.3˚±1.5˚) at symptomatic compared to the contralateral side (35.7±3.0, P<0.01, Table 1). Lateral recess height was also significant smaller on the symptomatic compared to the asymptomatic side (2.9±0.3 vs. 5.7±0.2 mm, P≤0.01). Accordingly, the area of the nerve root at the site of maximum compression was significantly smaller on the ipsilateral compared to the contralateral side (12.6±2.1 vs. 30.3±4.3, P≤0.01). No significant difference was detected in the area of the affected nerve at the mid-pedicle are of the caudal segment.
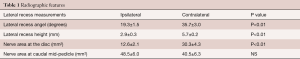
Full table
Surgical outcome
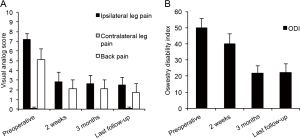
The mean operative blood loss was 14.6±2.7 mL and the mean operative time was 137.6±11.3 minutes. The level of surgery was L3/L4 in two patients (20%), L4/L5 in 6 patients (60%), and L5/S1 in the remaining two patients (20%, Table 2). The mean hospital stay was 0.8±0.3 days. Half of our patients were discharged home the same day of surgery. There were no intraoperative or perioperative complications. One patient experienced exacerbation of her back pain immediately after surgery but the pain had subsided at the 2 week follow up (0/10 VAS for back pain). At the time of last follow-up 12.6±1.7 months after the surgery, 8 out of 10 patients (80%) experienced a MCID improvement of their leg pain. Patients experienced significant improvement of the VAS for ipsilateral leg pain (preoperatively 7.2±0.5 vs. postoperatively 2.5±0.8, P=0.001, Figure 5). A similar degree of improvement was also recorded for VAS hip and buttock pain (preoperatively 6.8±0.9 vs. postoperatively 1.4±0.5, P<0.001). The VAS back pain also improved (preoperatively 5.1±1.1 vs. postoperatively 1.7±0.9, P<0.05). Prior to surgery patients were moderately disabled as indicated by an ODI (Oswestry Disability Index) of 50±5.8. At the time of last follow up, 7 out of 10 patients (70%) achieved a MCID for their ODI. Endoscopic lateral recess decompression resulted in a significantly improved ODI (22.2±5.1) compared to preoperative values (P=0.001).
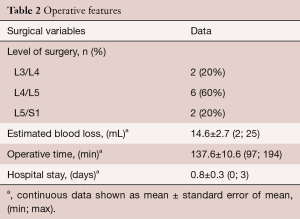
Full table
One patient, a 59-year-old male experienced persistent leg pain despite successful decompression of the lateral recess demonstrated on a repeat MRI. Given his history of heavy smoking a lower extremity ultrasound was obtained to rule out peripheral arterial disease. The patient is currently awaiting a diagnostic nerve root blocks to define the possible role of his severe left L4/5 and L5/S1 foraminal stenosis in causing his symptoms.
Discussion
In the current study we present radiographic diagnostic criteria, a highly targeted endoscopic decompression technique and early outcomes in patients with unilateral lateral recess stenosis.
While the literature on lateral recess stenosis is scant, in our clinical experience lateral recess stenosis is a prevalent diagnosis in patients with de novo lumbar radiculopathy or failed lumbar spine surgeries. Symptomatic motion-dependent nerve root compression occurs most commonly in the entrance zone of the lateral recess which is located between yellow ligament lined SAP posteriorly and the annulus of the disc anteriorly (5). Quantitative measurements of lateral recess stenosis have substantial intra- and inter-rater reproducibility (31,32) and correlate with pathological EMG recordings (33). In the current report we collected measurements of the lateral recess height and angle. Lateral recess height has been defined as the distance between the most medial point of superior articular facet and the posterior surface of vertebral body in most studies. Measurements of less than 2 mm (9), 3.6 mm (11), 4 mm (10) have been considered diagnostic for lateral stenosis. In the current report, the lateral recess height measurement was obtained at the medial margin of the traversing nerve within the lateral recess. However, the height of the symptomatic side (2.9±0.3 mm) was similar to the aforementioned studies (9-11). Lateral recess angle was proposed by a CT-based study to assess lateral recess stenosis. An angle of less than 30˚was considered highly indicative of stenosis (11). We adapted this CT-based approach for axial T2 weighted images with the angle placed tangential centered over the traversing nerve root. The lateral recess angle was 19.3˚±1.5˚ on the symptomatic side compared to 35.7˚±3.0˚ on the asymptomatic side. We hypothesize that our symptomatic lateral recess angle was narrower compared to CT-based measurements given that MRI also depicts yellow ligament and annular disc bulges. In patients with extremely narrow lateral recess angles we have observed intraoperatively that the traversing nerve root gets physically trapped. In this case it may be missed during the surgical decompression. We therefore always mobilize the lateral aspect of the supposed traversing nerve root. If it is not easily mobilized medially, there should be a high suspicion that the traversing nerve root might be trapped in the lateral recess. Accordingly, we hypothesize that once the lateral recess angle reaches a certain threshold, the traversing nerve root cannot freely move during motion of the spine but instead gets impinged.
Traditional surgical approaches recommended a wide laminectomy in combination with undercutting of the overhanging facet joints (34). However, partial removal of the facet joint may lead to joint instability and subsequent spondylolisthesis and scoliosis (35). Biomechanical studies have demonstrated that facet joints constrain axial rotation and flexion (36,37). Resection of facet joints destabilizes spinal segments proportional to the amount of joint removed (37-39). Moreover, removal of midline structures further contributes to segmental destabilization (38,40). In order to minimize post decompression instability and subsequent need for arthrodesis surgery, several minimally invasive technique aiming to preserve facet joint function and midline structures have been developed. The first step was the development of microscopic approaches that allowed to reduction of muscle detachment and isolated resection of the facet joint (41-44). These studies demonstrated the feasibility of isolated lateral recess decompression. Colak and colleagues presented microsurgical lateral recess decompression in 16 patients with bilateral lateral recess stenosis (45). The authors report an improvement of the preoperative VAS from 7.0 to 4.0 at 1 year follow up. The surgical corridor was further narrowed by the introduction of microendoscopic technique which was performed via a 16 mm tubular retractor (46). Hayashi and colleagues report on the use of microendoscopic decompression of lateral recess stenosis in 28 patients. At a mean follow-up time of 10.5 months VAS for radiculopathy improved from 6.5 to 1.1. The results of fully endoscopic spine surgery have been published for lateral recess stenosis (47). The authors included a total of 161 patients who were randomized either into microsurgical or endoscopic lateral recess decompression. The study found that both techniques resulted in similar symptomatic relief of leg pain, thus endoscopic decompression reduced the preoperative VAS for leg pain of 7.3 to 0.9 at 2 years follow-up. Importantly there was a significantly lower rate of progradient back pain in patients who underwent endoscopic compared to microscopic decompression.
To date, microscopic, microendoscopic and fully endoscopic minimally invasive techniques have been proposed for lateral recess decompression. Microsurgical decompression has the advantage of 3D visualization and the possibility to use large footprint tools such as Kerrison rongeurs for efficient decompression of neural structures. Microendoscopy provides are larger working corridor for the use of large footprint tools as well as a 25-degree angled visual field which allows undercutting of the facet joint. However, 2D visualization has the disadvantage of accurate depth measurement, hand-eye coordination, and poorer estimation of size in different depths of the field of view. Fully endoscopic surgery has the disadvantage of a small working corridor which makes removal of material tedious, a small field of view, as well as limitations of 2D visualization. The advantage of fully endoscopic surgery are a small working corridor with minimal irritation of the paraspinal muscles, constant irrigation which provides a clear operative field and gentle general retraction of the thecal sac and nerve roots as well as 25-degree angled view resulting in the ability to effectively undercut the fact joint. Further developments of tools for effective bone and soft tissue removal are necessary to improve the ability to decompress efficiently using a fully endoscopic technique.
The current study aims to propose radiographic lateral recess parameters on preoperative MRI that are associated with symptomatic lateral recess stenosis. There are number of limitations associated with this study. First, we have included a limited number of highly selected cases. These cases were specifically included to allow for measurement of radiographic lateral recess parameters on the non-symptomatic side to identify a threshold of these parameters that may be associated with radicular symptoms. All of our patients had a lateral recess angle of less than 25 degrees on their symptomatic side while the angle of asymptomatic side ranged from 25 to 52 degrees. Based upon these radiographic parameters we will recruit more patients to evaluate functional outcomes in a larger patient cohort. Moreover, one of our ten patients did not improve despite clear radiographic lateral recess stenosis, most likely due to co-existing foraminal stenosis. In order to improve our surgical success rate we now frequently refer patients to diagnostic nerve root blocks prior to decompression. However, patients with impingement of the same root in both lateral recess and next segment foramen remain a diagnostic and therapeutic challenge. We also acknowledge that our follow-up time is limited. Third, due to a lack of a control group we cannot make any assessment regarding how this technique compares to other surgical techniques. However, given that we used the interlaminar technique pioneered by Dr. Ruetten and colleagues, we expect our long-term results to be comparable (47).
The current report provides “symptomatic nerve centered” radiographic criteria to assess lateral recess stenosis, a highly focused surgical decompression technique and limited outcome data suggesting the feasibility of such an approach. Our radiographic criteria will be the foundation for recruitment of patients for future studies aiming to determine durability of symptomatic relief and possible benefits of minimal joint disruption.
Acknowledgements
The authors thank Kate Sweeney for graphical assistance.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All included patients provided written informed consent prior to undergoing the described procedures. Collection of perioperative and outcome data is part of the University of Washington Spine Care Quality Initiative.
References
- Kreiner DS, Shaffer WO, Baisden JL, et al. An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spinal stenosis (update) Spine J 2013;13:734-43. [Crossref] [PubMed]
- Deyo RA. Treatment of lumbar spinal stenosis: a balancing act. Spine J 2010;10:625-7. [Crossref] [PubMed]
- Treatment of Degenerative Lumbar Spinal Stenosis. Summary, Evidence Report/Technology Assessment: Number 32. AHRQ Publication No. 01-E047, March 2001. Agency for Healthcare Research and Quality, Rockville, MD.
- Taylor VM, Deyo RA, Cherkin DC, et al. Low back pain hospitalization. Recent United States trends and regional variations. Spine (Phila Pa 1976) 1994;19:1207-12; discussion 13. [Crossref] [PubMed]
- Lee CK, Rauschning W, Glenn W. Lateral lumbar spinal canal stenosis: classification, pathologic anatomy and surgical decompression. Spine (Phila Pa 1976) 1988;13:313-20. [Crossref] [PubMed]
- Arnoldi CC, Brodsky AE, Cauchoix J, et al. Lumbar spinal stenosis and nerve root entrapment syndromes. Definition and classification. Clin Orthop Relat Res 1976.4-5. [PubMed]
- Kirkaldy-Willis WH, Wedge JH, Yong-Hing K, et al. Lumbar spinal nerve lateral entrapment. Clin Orthop Relat Res 1982.171-8. [PubMed]
- Bartynski WS, Lin L. Lumbar root compression in the lateral recess: MR imaging, conventional myelography, and CT myelography comparison with surgical confirmation. AJNR Am J Neuroradiol 2003;24:348-60. [PubMed]
- Ciric I, Mikhael MA, Tarkington JA, et al. The lateral recess syndrome. A variant of spinal stenosis. J Neurosurg 1980;53:433-43. [Crossref] [PubMed]
- Mikhael MA, Ciric I, Tarkington JA, et al. Neuroradiological evaluation of lateral recess syndrome. Radiology 1981;140:97-107. [Crossref] [PubMed]
- Strojnik T. Measurement of the lateral recess angle as a possible alternative for evaluation of the lateral recess stenosis on a CT scan. Wien Klin Wochenschr 2001;113 Suppl 3:53-8. [PubMed]
- Lurie J, Tomkins-Lane C. Management of lumbar spinal stenosis. BMJ 2016;352:h6234. [Crossref] [PubMed]
- Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonoperative treatment for lumbar disc herniation: four-year results for the Spine Patient Outcomes Research Trial (SPORT). Spine 2008;33:2789-800. [Crossref] [PubMed]
- Boukebir MA, Berlin CD, Navarro-Ramirez R, et al. Ten-Step Minimally Invasive Spine Lumbar Decompression and Dural Repair Through Tubular Retractors. Oper Neurosurg (Hagerstown) 2017;13:232-45.
- Alimi M, Hofstetter CP, Torres-Campa JM, et al. Unilateral tubular approach for bilateral laminotomy: effect on ipsilateral and contralateral buttock and leg pain. Eur Spine J 2017;26:389-96. [Crossref] [PubMed]
- Mayer HM, List J, Korge A, et al. Microsurgery of acquired degenerative lumbar spinal stenosis. Bilateral over-the-top decompression through unilateral approach. Orthopade 2003;32:889-95. [Crossref] [PubMed]
- Katz JN, Lipson SJ, Larson MG, et al. The outcome of decompressive laminectomy for degenerative lumbar stenosis. J Bone Joint Surg Am 1991;73:809-16. [Crossref] [PubMed]
- Herno A, Airaksinen O, Saari T. Long-term results of surgical treatment of lumbar spinal stenosis. Spine 1993;18:1471-4. [Crossref] [PubMed]
- Getty CJ, Johnson JR, Kirwan EO, et al. Partial undercutting facetectomy for bony entrapment of the lumbar nerve root. J Bone Joint Surg Br 1981;63-B:330-5. [PubMed]
- Fox MW, Onofrio BM, Onofrio BM, et al. Clinical outcomes and radiological instability following decompressive lumbar laminectomy for degenerative spinal stenosis: a comparison of patients undergoing concomitant arthrodesis versus decompression alone. J Neurosurg 1996;85:793-802. [Crossref] [PubMed]
- Caputy AJ, Luessenhop AJ. Long-term evaluation of decompressive surgery for degenerative lumbar stenosis. J Neurosurg 1992;77:669-76. [Crossref] [PubMed]
- Benini A. Lumbar spinal stenosis. An overview 50 years following initial description. Orthopade 1993;22:257-66. [PubMed]
- Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976) 2000;25:2940-52; discussion 2952. [Crossref] [PubMed]
- Hägg O, Fritzell P, Nordwall A. The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur Spine J 2003;12:12-20. [PubMed]
- Fritz JM, Irrgang JJ. A comparison of a modified Oswestry Low Back Pain Disability Questionnaire and the Quebec Back Pain Disability Scale. Phys Ther 2001;81:776-88. [Crossref] [PubMed]
- Beurskens AJ, de Vet HC, Koke AJ, et al. Measuring the functional status of patients with low back pain. Assessment of the quality of four disease-specific questionnaires. Spine 1995;20:1017-28. [Crossref] [PubMed]
- Farrar JT, Young JP Jr, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94:149-58. [Crossref] [PubMed]
- Farrar JT, Portenoy RK, Berlin JA, et al. Defining the clinically important difference in pain outcome measures. Pain 2000;88:287-94. [Crossref] [PubMed]
- Leone A, Guglielmi G, Cassar-Pullicino VN, et al. Lumbar intervertebral instability: a review. Radiology 2007;245:62-77. [Crossref] [PubMed]
- Andreisek G, Deyo RA, Jarvik JG, et al. Consensus conference on core radiological parameters to describe lumbar stenosis - an initiative for structured reporting. Eur Radiol 2014;24:3224-32. [Crossref] [PubMed]
- Sipola P, Leinonen V, Niemelainen R, et al. Visual and quantitative assessment of lateral lumbar spinal canal stenosis with magnetic resonance imaging. Acta Radiol 2011;52:1024-31. [Crossref] [PubMed]
- Lurie JD, Tosteson AN, Tosteson TD, et al. Reliability of readings of magnetic resonance imaging features of lumbar spinal stenosis. Spine (Phila Pa 1976) 2008;33:1605-10. [Crossref] [PubMed]
- Kuittinen P, Sipola P, Aalto TJ, et al. Correlation of lateral stenosis in MRI with symptoms, walking capacity and EMG findings in patients with surgically confirmed lateral lumbar spinal canal stenosis BMC. Musculoskelet Disord 2014;15:247. [Crossref] [PubMed]
- Choudhury AR, Taylor JC. Occult lumbar spinal stenosis J Neurol Neurosurg Psychiatry 1977;40:506-10. [Crossref] [PubMed]
- Overdevest GM, Jacobs W, Vleggeert-Lankamp C, et al. Effectiveness of posterior decompression techniques compared with conventional laminectomy for lumbar stenosis. Cochrane Database Syst Rev 2015.CD010036. [PubMed]
- Hasegawa K, Kitahara K, Shimoda H, et al. Biomechanical evaluation of destabilization following minimally invasive decompression for lumbar spinal canal stenosis. J Neurosurg Spine 2013;18:504-10. [Crossref] [PubMed]
- Panjabi MM. Clinical spinal instability and low back pain. J Electromyogr Kinesiol 2003;13:371-9. [Crossref] [PubMed]
- Abumi K, Panjabi MM, Kramer KM, et al. Biomechanical evaluation of lumbar spinal stability after graded facetectomies. Spine (Phila Pa 1976) 1990;15:1142-7. [Crossref] [PubMed]
- Smith ZA, Vastardis GA, Carandang G, et al. Biomechanical effects of a unilateral approach to minimally invasive lumbar decompression. PLoS One 2014;9:e92611. [Crossref] [PubMed]
- Grunert P, Reyes PM, Newcomb AG, et al. Biomechanical Evaluation of Lumbar Decompression Adjacent to Instrumented Segments. Neurosurgery 2016;79:895-904. [Crossref] [PubMed]
- Mayer HM. Microsurgical Decompression of Acquired (Degenerative) Central and Lateral Spinal Canal Stenosis. In: Mayer HM. Editor. Minimally Invasive Spine Surgery. Berlin: Spinger, 2000.
- Goald HJ. Microlumbar discectomy: follow-up of 477 patients. J Microsurg 1980;2:95-100. [Crossref] [PubMed]
- Wilson DH, Kenning J. Microsurgical lumbar discectomy: preliminary report of 83 consecutive cases. Neurosurgery 1979;4:137-40. [Crossref] [PubMed]
- Caspar W. A new surgical procedure for lumbar disc herniation causing less tissue damage through a microsurgical approach. Adv Neurosurg 1977;4:74-7. [Crossref]
- Colak A, Topuz K, Kutlay M, et al. A less invasive surgical approach in the lumbar lateral recess stenosis:direct approach to the medial wall of the pedicle. Eur Spine J 2008;17:1745-51. [Crossref] [PubMed]
- Hayashi A, Oshima Y, Shiboi R, et al. Microendoscopic Posterior Decompression for the Treatment of Lumbar Lateral Recess Stenosis. J Spine 2016;5:317. [Crossref]
- Ruetten S, Komp M, Merk H, et al. Surgical treatment for lumbar lateral recess stenosis with the full-endoscopic interlaminar approach versus conventional microsurgical technique: a prospective, randomized, controlled study. J Neurosurg Spine 2009;10:476-85. [Crossref] [PubMed]


