Unusual delayed presentation of superior mesenteric artery syndrome following scoliosis correction surgery—a case report and review of literature
Introduction
Superior mesenteric artery (SMA) syndrome was first described by Boernerus as early as 1752 and then subsequently by Rokitansky in 1842 (1-3). Since then, several names such as cast syndrome (4), arteriomesenteric duodenal obstruction (5), SMA syndrome (6) and Wilkie syndrome (7) have been adopted by different investigators to describe this condition. It has been associated with many predisposing conditions like post spinal deformity surgery; sudden weight loss (dietary disorders, trauma, postoperative states); chronic wasting disease; etc., When SMA originates at an acute angle from aorta, it can potentially lead to compression of third part of duodenum causing symptoms and signs of small bowel obstruction. In addition, mortality rate can be as high as 33% due to fatal complications like aspiration pneumonia, acute gastric rupture and cardiovascular collapse (8-11). In this case report, we present a 13-year-old patient who was diagnosed with SMA syndrome in the late post-operative period (5.1 weeks) following scoliosis correction surgery. Such late presentations are very uncommon.
Case presentation
Our patient is a 13-year-old female student, was first diagnosed with Idiopathic Scoliosis at the age of 5 years during a routine school screening program. She was initially treated with observation and subsequently with a Boston brace when the curve progressed. The curve continued to progress despite brace treatment. At the age of 13 years, the decision was made for surgery when the Cobb angle had worsened into the operative range. She had no premorbid medical or surgical illness except for the diagnosis of Kawasaki disease made during infancy period for which no active treatment was instituted. She attained menarche at 11 years of age and had no family history of scoliosis. Preoperatively, patient weighed 42 kg (31 percentile) with a height of 1.58 meter (54 percentile) and body mass index (BMI) of 16.4 (21 percentile). She had a thoracolumbar truncal rotation of 22 degrees with truncal listing of 4 cm to the right. Whole Spine EOS Radiograph and Bending (right and left) Radiographs were suggestive of a Lenke 6CN curve with a right thoracolumbar curve (T8–L3) measuring 63 degrees bending to 39 degrees; left thoracic curve (T2–T8) of 33 degrees bending to 27 degrees and thoracic kyphosis (T5–T12) measuring 29 degrees (Figure 1). She was Risser grade 4. Preoperative workup including blood tests, pulmonary function tests and MRI were within normal limits.
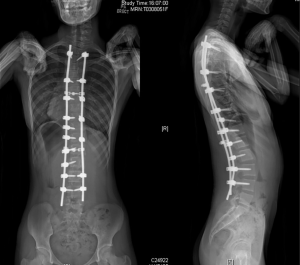
She underwent posterior spinal instrumentation and fusion from T3 to L4 (Figure 2). Postoperatively patient had a height gain of 5 cm with improvement in trunk length from 34 to 39 cm and an excellent curve correction. She had an uneventful postoperative period and was discharged on the fourth postoperative day. She was subsequently reviewed on post-operative day 14 and reported no unusual symptoms.
At 5.1 weeks postoperatively, patient presented to the emergency department with complaints of colicky abdominal pain improving when sitting forward, for duration of 10 days and vomiting for 2 days following food intake. Despite a good appetite, she was vomiting after every meal. In addition, she also reported reduced bowel output. On examination, she was dehydrated with tenderness over the epigastric region. Bowel sounds were present. She had 4 kg loss of body weight from 41 to 38 kg in 5 weeks’ time.
The patient was admitted for further evaluation. She was kept nil by mouth and administered intravenous fluids and antiemetics. Both plain radiographs and ultrasound did not reveal any abnormal findings. A barium meal and follow-through was done with the help of 100 mL of water soluble contrast (Omnipaque-350) which revealed a dilatation (4 cm) and stasis of contrast in third segment of duodenum (D3) (Figure 3). The contrast seemed to flow smoothly with the patient in left lateral decubitus position.

A provisional diagnosis of Superior Mesenteric Syndrome was made. She improved symptomatically with conservative management with nasojejunal tube insertion initially and then gradual restarting of oral feeds from day 4 of admission. She was able to tolerate protein rich fluid followed by gradual intake of solids.
The patient was successfully discharged from hospital after 4 days of observation. She was reviewed subsequently in the outpatient clinic 8 weeks after discharge and was noted to be symptom-free. The patient and her family were informed that data from this case would be submitted for publication, and gave their consent.
Discussion
SMA Syndrome is defined as prolonged postoperative nausea for more than one week and vomiting associated with an ileus, requiring supplemental nutrition coupled with radiological confirmation of constriction of third part of duodenum and delayed gastric emptying (12). It is an uncommon cause of intestinal obstruction due to extrinsic compression of third part of duodenum as it passes between the SMA anteriorly and the aorta and vertebral column posteriorly. The third part of duodenum is surrounded by the mesenteric fat pad and lymphatic tissue in the interval between SMA and aorta and can potentially behave as a “nutcracker” when this delicate relationship gets disturbed (3,13). The development of SMA syndrome is closely related to the Aorta-SMA angle and the Aorta-SMA distance which are usually between 45 to 60 degrees and 13 to 34 mm respectively as shown by angiogram studies (10). An Aorta-SMA angle <22 to 25 degrees and Aorta-SMA distance <8 mm is likely to cause symptoms of SMA syndrome (14,15). Various factors like asthenic body habitus, exaggerated lumbar lordosis, extended supine positioning (post hip spica application) and lengthening of spinal column following spinal deformity surgery can cause reduction in Aorta-SMA angle (3,16,17). In addition, depletion of mesenteric fat pad around duodenum as seen in cachexia following malignancy, malabsorption syndromes, anorexia nervosa, burns, polytrauma and postoperative state can also cause SMA syndrome.
SMA syndrome gets usually manifested between 6 to 8 days following surgery (50%). Classically, the most common presentation is the vomiting (92.9%) which can be bilious or non-bilious, followed by abdominal pain / tenderness (57.1%) (18). Other features may include epigastric pain, postprandial bloating and abdominal tenderness with no or minimal rigidity on examination. The degree of obstruction can vary from partial to complete depending on the extent of extrinsic compression and mucosal edema. Complete obstruction result in dehydration, electrolyte imbalance, oliguria, shock and even death has been reported. SMA syndrome often sets off a vicious cycle where frequent vomiting and feed intolerance lead to weight loss which causes further narrowing of Aorta-SMA angle, thus aggravating the duodenal compression and resultant vomiting. Hence, management of SMA should be aimed at breaking this vicious cycle.
Plain radiographs are usually unremarkable except some gastroduodenal distension with relative absence of gas in the distal bowel. Barium meal with follow-through imaging is the investigation of choice which can demonstrate abrupt cut off of contrast in third duodenal segment (D3) and delayed gastric emptying (3,19). In addition, postural change (lateral decubitus) might relieve obstruction. Treatment of SMA includes intravenous fluids, nil by mouth, nasoduodenal tube decompression, antispasmodics and parenteral nutrition in selected cases (20). Simple postural changes like knee chest position, left lateral decubitus position and upright position may facilitate decompression (21). Frequent small feedings and if necessary total parenteral hyperalimentation will restore the retroperitoneal fat, thereby increasing the Aorta-SMA distance (21). Additional treatment strategies include strengthening of lax abdominal musculature to correct exaggerated lumbar lordosis. When non-operative treatment fails, surgical intervention is required to relieve obstruction. The procedure of choice is side to side duodenojejunostomy. Other procedures include gastrojejunostomy, Strong’s procedure, and Ladd procedure (18).
SMA syndrome following scoliosis surgery is due to reduction of Aorta-SMA angle and Aorta-SMA distance following lengthening of vertebral column. Other predisposing factors like asthenic body habitus, malnourishment, etc., can further reduce the already small Aorta-SMA angle and distance. Incidence of SMA syndrome following scoliosis surgery is roughly between 1% to 4.7% (18,22) and most commonly occur within the first week with an average 6 to 12 days (18,23). Late presentation of SMA syndrome is very uncommon and very few cases have been published in the English literature to date. Kennedy and Cooper et al., reported SMA syndrome at postoperative day 40 in a 14-year-old male after scoliosis correction using Harrington instrumentation and Body cast which progressed rapidly to death (24). Tsirikos et al., described SMA syndrome in a 16-year-old female at 45 days following anterior spinal fusion and instrumentation, who had symptomatic improvement after conservative management (25). Thus, a high index of suspicion is required even years after scoliosis surgery (26). Braun et al., identified the following predictive factors for the development of SMA syndrome in scoliosis patients: (I) BMI <25 percentile for the age; (II) stiffer thoracic curve (correction of less than 60% on bending, laterally shifted curve with either Lenke B or C); (III) thoracoplasty; (IV) anterior approach; (V) staged operation (12). In this case report, we retrospectively identified the following predictive factors—(I) low preoperative BMI (15.9 percentile) which further dropped (6.4 percentile) at the time of presentation due to perioperative weight loss; (II) Lenke C curve; (III) stiffer thoracic curve (correction to only 85% on bending).
Conclusions
It is essential that we identify high risk patients preoperatively so that we could optimize them with proper intensive dietary supplementation. Postoperatively, a high index of suspicion needs to be retained to identify this syndrome at an early stage so that conservative management may be initiated with good clinical outcome. SMA syndrome can be potentially life threatening when the diagnosis is missed or delayed.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Evarts CM, Winter RB, Hall JE. Vascular compression of the duodenum associated with the treatment of scoliosis. J Bone Joint Surg Am 1971;53:431-44. [Crossref] [PubMed]
- Rokitansky C. Handbuch der pathologischen Anatomie, 1st ed. Wien: Braunmüller & Seidel, 1842:187.
- Derincek A, Wood KB, Muench CA. Superior Mesenteric Artery Syndrome Following Correction of Kyphosis in an Adult. J Spinal Disord Tech 2004;17:549-53. [Crossref] [PubMed]
- Dorph MH. The cast syndrome. N Engl J Med 1950;243:440-2. [Crossref] [PubMed]
- Strong EK. Mechanics of arteriomesenteric duodenal obstruction and direct surgical attack upon etiology. Ann Surg 1958;148:725-30. [Crossref] [PubMed]
- Jones PA, Wastell C. Superior mesenteric artery syndrome. Postgrad Med J 1983;59:376-9. [Crossref] [PubMed]
- Kwan E, Lau H, Lee F. Wilkie’s syndrome. Surgery 2004;135:225-7. [Crossref] [PubMed]
- Boseker EH, Moe JH, Winter RB, et al. Determination of “normal” thoracic kyphosis: a roentgenographic study of 121 “normal” children. J Pediatr Orthop 2000;20:796-8. [Crossref] [PubMed]
- Zhu ZZ, Qui Y. Superior mesenteric artery syndrome following scoliosis surgery: its risk indications and treatment strategy. World J Gastroenterol 2005;11:3307-10. [Crossref] [PubMed]
- Crowther MA, Webb PJ, Eyre-Brook IA. Superior mesenteric artery syndrome following surgery for scoliosis. Spine (Phila Pa 1976) 2002;27:E528-33. [Crossref] [PubMed]
- Sapkas G, O'Brien JP. Vascular compression of the duodenum (cast syndrome) associated with the treatment of spinal deformities. A report of six cases. Arch Orthop Trauma Surg 1981;98:7-11. [Crossref] [PubMed]
- Braun SV, Hedden DM, Howard AW. Superior mesenteric artery syndrome following spinal deformity correction. J Bone Joint Surg Am 2006;88:2252-7. [PubMed]
- Vitale MG, Higgs GB, Liebling MS, et al. Superior mesenteric artery syndrome after segmental instrumentation: a biomechanical analysis. Am J Orthop (Belle Mead NJ) 1999;28:461-7. [PubMed]
- Qian BP, Ji ML, Jiang J, et al. Anatomic relationship between superior mesenteric artery and aorta before and after surgical correction of thoracolumbar kyphosis. J Spinal Disord Tech 2013;26:E293-8. [Crossref] [PubMed]
- Ylinen P, Kinnunen J, Hockerstedt K. Superior mesenteric artery syndrome. A follow-up study of 16 operated patients. J Clin Gastroenterol 1989;11:386-91. [Crossref] [PubMed]
- Munns SW, Morrissy RT, Golladay ES, et al. Hyperalimentation for superior mesenteric-artery (cast) syndrome following correction of spinal deformity. J Bone Joint Surg Am 1984;66:1175-7. [Crossref] [PubMed]
- Hutchinson DT, Bassett GS. Superior mesenteric artery syndrome in pediatric orthopedic patients. Clin Orthop Relat Res 1990.250-7. [PubMed]
- Lam DJ, Lee JZ, Chua JH, et al. Superior mesenteric artery syndrome following surgery for adolescent idiopathic scoliosis: a case series, review of the literature, and an algorithm for management. J Pediatr Orthop B 2014;23:312-8. [Crossref] [PubMed]
- Griffiths GJ, Whitehouse GH. Radiological features of vascular compression of the duodenum occurring as a complication of the treatment of scoliosis (the cast syndrome). Clin Radiol 1978;29:77-83. [Crossref] [PubMed]
- Barner HB, Sherman CD. Vascular compression of the duodenum. Int Abstr Surg 1963;117:103-18. [PubMed]
- Hines JR, Gore RM, Ballantyne GH. Superior mesenteric artery syndrome, Diagnostic criteria and therapeutic approaches. Am J Surg 1984;148:630-2. [Crossref] [PubMed]
- Tsirikos AI, Jeans LA. Superior mesenteric artery syndrome in children and adolescents with spine deformities undergoing corrective surgery. J Spinal Disord Tech 2005;18:263-71. [PubMed]
- Altiok H, Lubicky JP, Dewald CJ, et al. The Superior Mesenteric Artery Syndrome in Patients with Spinal Deformity. Spine(Phila Pa 1976) 2005;30:2164-70. [Crossref] [PubMed]
- Kennedy RH, Cooper MJ. An Unusually severe case of the cast syndrome. Postgrad Med J 1983;59:539-40. [Crossref] [PubMed]
- Tsirikos AI, Anakwe RE, Baker AD. Late presentation of superior mesenteric artery syndrome following scoliosis surgery: a case report. J Med Case Rep 200819;2:9.
- Abol Oyoun N, Kadhim M, Dormans JP. Late-onset superior mesenteric artery syndrome four years following scoliosis surgery - a case report. SICOT J 2015;1:12. [Crossref] [PubMed]