Subsidence following anterior lumbar interbody fusion (ALIF): a prospective study
Introductions
Vertebral interbody fusion is a surgical intervention commonly undertaken in selected patients with discogenic pain and/or mechanically unstable spinal levels (1,2). The most common location for interbody fusion is in the lumbar spine, as this region experiences the most mechanical strain and is most prone to pathology (1). Although multiple approaches can successfully achieve interbody fusion, an anterior surgical approach is widely accepted as having an acceptable success rate with few complications (1,3,4).
Anterior lumbar interbody fusion (ALIF) has become a widely utilized surgical intervention across the world. Known complications have been studied in ALIF and correlated with potential clinical outcomes. One of these potential complications is the subsidence of disc height in the post-operative period (5-7). Previous research has implied that disc height is increased acutely following surgery as a factor of operative distraction, but decreases over the next weeks to months (6). Generally the disc height has not been shown to decrease to less than the preoperative disc height, however this has been documented in some cases (6,8,9). The time frame of subsidence has been reported as varying from 1 week to 8 months, presenting a large, non-specific time window (9).
Although it was previously concluded that implant subsidence was not correlated with clinical outcome, no trials have consequently shown that mechanical stability is unaffected by subsidence, which is empirically of concern. The primary hypothesis of this paper is that subsidence is more prevalent than previously believed, being seen in up to 100% of ALIF cases, and is an acceptable aspect of the progression to fusion with this technique.
Disc height has previously been estimated using a variety of techniques, all of which use substitute measurements to extrapolate an average disc height. Proxy measurements have included: average disc height using the most anterior and most posterior points of adjacent end-plates, mid-point height measured perpendicular to the inferior endplate, and measurement of the mid-point distance from the inferior endplate of the level above, to the inferior endplate of the level in question (10-12).
This study measured disc heights in 147 patients operated from 2011 to 2013 by the same surgical team, pre-operatively, immediately post-operatively, 6 weeks post-operatively and 6 months postoperatively and/or most recent scan. To the authors’ knowledge, this is one of the largest and most extensive assessment of subsidence in ALIF literature to date.
Methods
Results were obtained by reviewing scans of 147 patients, all of whom underwent surgery by the same senior neurosurgeon across two hospitals. Disc heights were measured on CT scans taken pre-operatively, immediately post-operatively, on erect X-rays at 6 weeks post-operatively, CT scans at 6-month-postoperatively when evaluating fusion and using the latest available CT scan performed as clinically indicated, such as suspicion of incomplete fusion. Clearance for the prospective study was obtained through the Human Research Ethics Committee of New South Wales Health (reference No. 11/183). Patients undergoing ALIF surgery were included in the study with indications: degenerative disc disease without radiculopathy, degenerative disc disease with radiculopathy, spondylolisthesis, failed posterior fusion, adjacent segment disease and scoliosis requiring correction. Exclusion criteria were patients with concurrent local or systemic infection, neoplasia, significant cardiac disease, fever (>38.5 °C), or metal allergy; as well as patients who were pregnant or breast-feeding, who were mentally incompetent, who had a history of alcohol or drug abuse, and who were at increased risk of vascular or bowel complications related to the anterior approach.
Patients received stand-alone PEEK integral cage devices. In particular, the vast majority (89.1%) received the SynFix-LR PEEK integral cage device (DePuy Synthes, West Chester, PA, USA) with four diverging intrinsic screws and anterior locking plate, without anterior tension band plating nor posterior instrumentation. The implant sizing varied across patients in accordance with the disc height of neighbouring healthy lumbar discs, ranging from 12–19 mm height with either 8° or 12° lordotic angle to ensure sufficient distraction. Bone graft substitute i-FACTOR (Cerapedics, Westminster, CO, USA) was used for 136 patients and is comprised of anorganic bone matrix bound to anorganic P-15 small peptide, together facilitating attachment of osteogenic cells. For the remaining 11 patients, recombinant human bone morphogenetic protein 2 (rhBMP2) (Medtronic Sofamor Danek, Memphis, TN, USA) was used. In 7 (4.8%) patients there had been a previous fusion performed and no patients required additional posterior pedicle screw fixation to augment ALIF.
The radiological parameters for subsidence and fusion were measured by a neurosurgeon (P.R.). Fusion rates were assessed using reconstructed axial and coronal fine-cut CT scans. Criteria for established fusion were bridging trabecular formation across the intervertebral disc space with the absence of radiolucency spanning more than half of the implant. The anterior and posterior intervertebral disc heights were measured and averaged. Endplate levels were taken as a straight-line average of the endplate as seen on the most central image in all planes, using the most anterior and posterior points excluding osteophytes. Osteophytes were identified as superficial extrusions of bone anteriorly or posteriorly beyond the main vertebral body. This allows for reliable disc height estimation without being confounded by central disc erosion. However, it can be a difficult measurement in images with significant anterolisthesis, retrolisthesis, or osteophyte formation. The local disc angle (LDA) was determined by the angle formed by the intersection of the inferior endplate line and the superior endplate line of the index disc level. Lumbar lordosis (LL) was measured between the superior endplate of L-1 to the superior endplate of S-1 using the Cobb method.
Subsidence was defined as greater than or equal to 2 mm loss of height. Early subsidence was defined as occurring within the first 6 weeks postoperatively and delayed subsidence as after 6 weeks postoperatively. Cranial (inferior endplate) subsidence was classed as type 2 and caudal (superior endplate) was considered type 1 subsidence as per Malham et al.’s study (11).
Clinical outcome was measured preoperatively and postoperatively using the Oswestry Disability Index (ODI) and the Visual Analog Scale (VAS) back pain score. Questionnaire data from the Short Form 12 Item survey (SF-12) were compiled in a custom-designed database. Preoperative and postoperative study outcomes were examined with analysis of variance and repeated-measures general linear models adjusted for age and sex. Analyses were based on 2-sided tests with values of P<0.05 considered significant with Bonferroni correction when appropriate. Correlation studies were performed using Pearson’s coefficients to investigate relations between changes in radiologic parameters and improvements in VAS, ODI, and SF-12 scores. Data analysis and statistical evaluation was conducted using IBM SPSS Statistics 22 (IBM Corporation, Armonk, NY, USA).
Results
Patient demographics
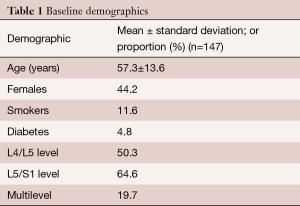
A total of 147 patients underwent ALIF in our study. The mean age of the study group was 57.3±13.6 years. Sixty-five of the patients were female (44.2%). From the total cohort, 17 patients (11.6%) were smokers and 7 patients (4.8%) had diabetes. Only one patient used corticosteroids, due to comorbid Crohn’s disease. 74 patients (50.3%) underwent ALIF at the L4/L5 level, 95 patients (64.6%) at the L5/S1 level and 29 patients (19.7%) underwent multilevel fusion (Table 1). Indications for ALIF are detailed in Table 2.
Full table
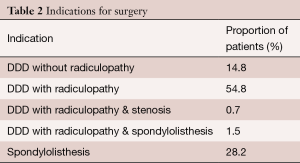
Full table
Operative outcomes
Fifteen patients (10.2%) demonstrated subsidence of mean 4.7 mm (range, 2.4–7.8). These were all delayed cage subsidence (DCS).The mean age of the patients with subsidence was 67 years and the male to female ratio was equivocal (7:8). Four of these patients were overweight and another had pseudoarthrosis.
There were 15 patients with type 1 (caudal endplate) subsidence; 3 with anterior, 2 with posterior and 10 with both anterior and posterior subsidence. No patients had type 2 (cranial endplate) subsidence alone, but 2 patients had both type 1 and type 2 subsidence.
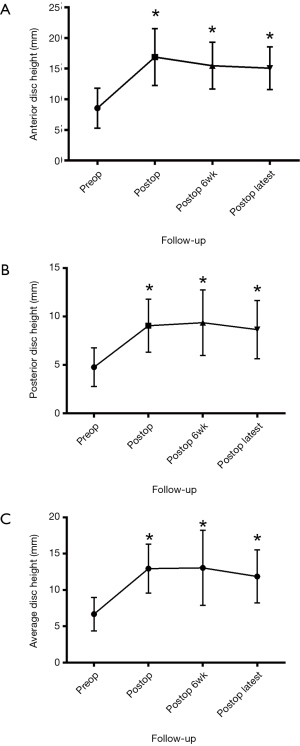
The preoperative anterior disc height was 8.6±0.4 mm, which improved to 16.9±0.4 mm immediately post operatively, 15.5±0.5 mm at 6-week follow-up, and 15.1±0.5 mm at latest follow-up. For posterior disc height, preoperatively this was 4.7±0.2 mm, which improved to 9.1±0.3 mm immediately postoperatively, 9.4±0.4 mm at 6-week follow-up, and 8.7±0.4 mm at latest follow-up (Figure 1).
The 91.2% (n=114/125) of patients with appropriate radiological follow-up demonstrated fusion by latest follow-up whilst 11 patients did not demonstrate fusion. Appropriate CT scans at 6 months were not available for 22 patients. In our study, only 19.7% of patients received multilevel ALIF, that is, ALIF at two or more adjacent levels. Of these, only 2 patients received three-level ALIF, for which all segments fused, and only 1 received four-level ALIF, for which only immediate postoperative follow-up was available.
The mean LL angle was 42.5°±10.8° and the mean LDA was 6.7°±4.0°. The mean cage height, length and width was 13.4±1.4, 37.8±1.6 and 30.0±0.4 mm respectively. The mean cage lordosis was 9.0°±1.8°.
Functional outcomes
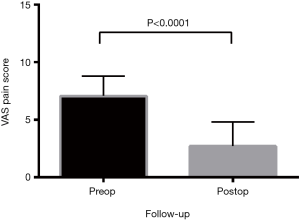
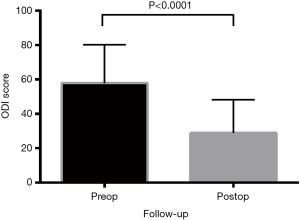
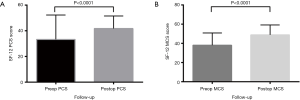
All functional outcomes demonstrated improvement, with reduction in VAS and ODI scores and increase in SF-12 scores. Preoperative VAS pain score was mean 7.1±0.2, reducing post-operatively to 2.7±0.2 (P<0.0001) (Figure 2). The preoperative ODI was also significantly reduced post-operatively (P<0.0001) from mean 57.8±2.0 to 28.8±1.8 (Figure 3). Preoperative SF-12 physical component summary (PCS) was 33.2±1.7, which was increased to 41.7±0.9 post-operatively (P<0.0001). Similarly, preoperative SF-12 mental component summary (MCS) score was 38.0±1.2, and increased to 48.9±1.0 post-operatively (P<0.0001) (Figure 4).
Correlation analysis
No significantly association was found between subsidence rates and changes in VAS (P=0.36), ODI (P=0.55), SF-12 PCS (P=0.69) or SF-12 MCS (P=0.64). There was also no significant association between degree of subsidence with fusion rates (P=0.85). In our cohort, we did not find significant correlations between subsidence and demographic factors including age, sex, spondylolisthesis, steroid use, diabetes, smoking, BMI or LDA. There was a significant negative correlation between LL with extent of subsidence (Pearson correlation =−0.754, P=0.012), i.e., the greater the LL correction, the lower the extent of subsidence on follow-up.
Discussion
An anterior approach for lumbar fusion is a procedure used to treat spine instability with the goal of providing a solid and lasting union of the affected vertebrae (1,10,13,14). An anterior approach to the spine, as opposed to a posterior approach, does not disturb the paravertebral muscles and ligaments, which are involved in spine mobility and stability (15). As a result there is improved spinal mobility and decreased muscle pain postoperatively in the anterior approach (16). Anterior exposure also provides greater surgical versatility and exposure intraoperatively (1). In addition, ALIF has been shown to be effective in decompressing the intervertebral foramen and restoring disc interspace height (9).
A complication that can arise from ALIF is subsidence, which is a decrease in the vertical height of the vertebral disc space prior to complete fusion. Subsidence is an important consideration as the reduction in disc space can detrimentally affect mechanical correction and clinical outcomes (10). In the present study, we found that the overall subsidence rate at follow up was generally low following ALIF for this patient series. In the patients where subsidence was observed it was mostly attributed to the caudal endplate. There was no significant correlation between degree of subsidence with changes in clinical outcomes including VAS back pain, ODI, SF-12 PCS and MCS scores or fusion rates. This result is broadly consistent with prior published studies focusing on posterior lumbar interbody fusion (17-20). Collectively, these results suggest that bony fusion as well as patient reported measures of pain and disability were not significantly impaired by subsidence of the ALIF cage into the vertebral body.
A number of factors have been proposed to influence subsidence rates. Inappropriate intraoperative endplate preparation of the vertebral bodies with extensive endplate resection has been suggested as one factor contributing to subsidence (21,22). However, endplate preparation can be controlled in ALIF as there is direct visualization of the disc space and removal of the ALL creates a non-rigid space during disc preparation. Marchi et al. demonstrated that in stand-alone lateral interbody fusion, wide cages avoid subsidence and restore segmental lordosis to a greater extent (23). Furthermore, it has been found that ALIF conducted solely using bone grafts can result in high subsidence rates of up to 100% (6,8). Metal interbody cages have thus been used as an alternative as they can better maintain disc space height during the process of bone graft incorporation into the fusion mass (9). Sandhu et al. (24) found that subsidence in sheep was reduced when a threaded cage was used as opposed to an iliac crest bone graft. Further clinical studies have also found lower subsidence rates when using a metal interbody cage in ALIF (9,10,25). In the present study, we also found that improved correction of segmental LL was significantly associated with reduced subsidence extent on follow-up, which further supports the notion of adequate lordosis correction during stand-alone ALIF.
More recently, non-absorbable, biocompatible PEEK cages have been used as they are radiolucent and have similar mechanical properties to bone (16,26,27). The radiolucency of this PEEK cage material has allowed for improved ease and clarity during assessment of fusion (28,29). The PEEK cages have also been proposed to have load-bearing properties superior to metal cages, and thus can further reduce subsidence rates (16). This would support the results of our current study, which found a low 8.7% rate of subsidence in standalone ALIF using PEEK cages. In the current study, the use of cages with a modulus of elasticity close to bone in our cohort receiving stand alone ALIF resulted in significantly improved functional outcomes with reduced VAS and ODI scores along with higher SF-12 MCS and PCS scores post-operatively.
In terms of the site of implant subsidence, the predominated location was found to be in the caudal superior endplate. This is most likely due to variations in strength across endplate regions (9). In a study using human cadavers, Grant et al. (30) found that the posterior region of the endplate was stronger than the anterior region, the vertebral periphery was stronger than the center and the caudal inferior endplate was approximately 40% stronger than the cervical superior endplate. The results of this biomechanical study are consistent with not only our findings but also that of Choi et al. (9), who reported a subsidence rate of 39.1% at the superior endplate and 17.3% at the inferior endplate. Kumar et al. (31) also found greater subsidence rates at the posterior endplate, supporting the findings of Grant et al. (11,30). Variations in bone mineral density have been shown to play a role in subsidence and thus could potentially explain the differences in bone strength at various regions of the endplate (32).
All the patients in this study developed DCS, which was defined as occurring at greater than 6 weeks post-operatively. This finding was consistent with that of Choi et al. (9), who reported a median period for cage subsidence at 2.75 months. Cheung et al. (8) also reported onset of subsidence mostly within the first 3 months after ALIF using an iliac bone graft. However, Kumar et al. (31) found that subsidence occurred within 15 days when using a femoral bone graft. Thus, there are large variations in subsidence onset time reported in the literature when using both interbody cages and bone grafts (8,9,31). The large variations in subsidence onset as reported in the literature may be due to variations in patient BMI between studies. A higher BMI has been associated with greater subsidence as it can accelerate disc degeneration and increase spinal instability (33). Differences in cage design have also been shown to have an effect on the strength of the implant and thus its ability to resist subsidence (34). Additionally, surgical technique has also been implicated, with rigorous endplate preparation resulting in loss of bony endplate, leading to subsidence.
It is important to recognize the limitations of this study (35). Firstly, there was significant heterogeneity in the study due to usage of different graft materials along with innate variations in the included patient population. Although bone mineral density has been implicated as a potential contributor to subsidence, this was not measured nor controlled for in our study (32). The patient series was also from a single spine surgeon, limiting the external validity of the findings despite allowing consistency in surgical technique. Nevertheless our study is the largest prospective study investigating the subsidence in ALIF to date. Further, the measurement of subsidence was conducted using fine cut CT scans and the technique was standardized across all patients.
Conclusions
In conclusion, we found that the subsidence rate at follow-up was generally low following standalone ALIF for this patient series. In the patients where subsidence was observed, it was mostly attributed to the caudal endplate. There was no significant correlation found between degree of subsidence with improvements in clinical outcomes in terms of VAS, ODI and SF-12 scores or fusion rates.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Human Research Ethics Committee of New South Wales Health (No. 11/183) and written informed consent was obtained from all patients.
References
- Ikard RW. Methods and complications of anterior exposure of the thoracic and lumbar spine. Arch Surg 2006;141:1025-34. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2-18. [PubMed]
- Mobbs RJ, Phan K, Daly D, et al. Approach-Related Complications of Anterior Lumbar Interbody Fusion: Results of a Combined Spine and Vascular Surgical Team. Global Spine J 2016;6:147-54. [Crossref] [PubMed]
- Phan K, Thayaparan GK, Mobbs RJ. Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion--systematic review and meta-analysis. Br J Neurosurg 2015;29:705-11. [Crossref] [PubMed]
- Chen D, Fay LA, Lok J, et al. Increasing neuroforaminal volume by anterior interbody distraction in degenerative lumbar spine. Spine (Phila Pa 1976) 1995;20:74-9. [Crossref] [PubMed]
- Dennis S, Watkins R, Landaker S, et al. Comparison of disc space heights after anterior lumbar interbody fusion. Spine (Phila Pa 1976) 1989;14:876-8. [Crossref] [PubMed]
- Weiner BK, Fraser RD. Spine update lumbar interbody cages. Spine (Phila Pa 1976) 1998;23:634-40. [Crossref] [PubMed]
- Cheung KM, Zhang YG, Lu DS, et al. Reduction of disc space distraction after anterior lumbar interbody fusion with autologous iliac crest graft. Spine (Phila Pa 1976) 2003;28:1385-9. [Crossref] [PubMed]
- Choi JY, Sung KH. Subsidence after anterior lumbar interbody fusion using paired stand-alone rectangular cages. Eur Spine J 2006;15:16-22. [Crossref] [PubMed]
- Beutler WJ, Peppelman WC Jr. Anterior lumbar fusion with paired BAK standard and paired BAK Proximity cages: subsidence incidence, subsidence factors, and clinical outcome. Spine J 2003;3:289-93. [Crossref] [PubMed]
- Malham GM, Parker RM, Blecher CM, et al. Assessment and classification of subsidence after lateral interbody fusion using serial computed tomography. J Neurosurg Spine 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Rao PJ, Maharaj MM, Phan K, et al. Indirect foraminal decompression after anterior lumbar interbody fusion: a prospective radiographic study using a new pedicle-to-pedicle technique. Spine J 2015;15:817-24. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Thayaparan GK, et al. Anterior Lumbar Interbody Fusion as a Salvage Technique for Pseudarthrosis following Posterior Lumbar Fusion Surgery. Global Spine J 2016;6:14-20. [Crossref] [PubMed]
- Rao PJ, Ghent F, Phan K, et al. Stand-alone anterior lumbar interbody fusion for treatment of degenerative spondylolisthesis. J Clin Neurosci 2015;22:1619-24. [Crossref] [PubMed]
- Quraishi NA, Konig M, Booker SJ, et al. Access related complications in anterior lumbar surgery performed by spinal surgeons. Eur Spine J 2013;22 Suppl 1:S16-20. [Crossref] [PubMed]
- Behrbalk E, Uri O, Parks RM, et al. Fusion and subsidence rate of stand alone anterior lumbar interbody fusion using PEEK cage with recombinant human bone morphogenetic protein-2. Eur Spine J 2013;22:2869-75. [Crossref] [PubMed]
- Swan J, Hurwitz E, Malek F, et al. Surgical treatment for unstable low-grade isthmic spondylolisthesis in adults: a prospective controlled study of posterior instrumented fusion compared with combined anterior-posterior fusion. Spine J 2006;6:606-14. [Crossref] [PubMed]
- Lequin MB, Verbaan D, Bouma GJ. Posterior lumbar interbody fusion with stand-alone Trabecular Metal cages for repeatedly recurrent lumbar disc herniation and back pain. J Neurosurg Spine 2014;20:617-22. [Crossref] [PubMed]
- Tokuhashi Y, Ajiro Y, Umezawa N. Subsidence of metal interbody cage after posterior lumbar interbody fusion with pedicle screw fixation. Orthopedics 2009.32. [PubMed]
- Hentenaar B, Spoor AB, de Waal Malefijt J, et al. Clinical and radiological outcome of minimally invasive posterior lumbar interbody fusion in primary versus revision surgery. J Orthop Surg Res 2016;11:2. [Crossref] [PubMed]
- Hwang SL, Hwang YF, Lieu AS, et al. Outcome analyses of interbody titanium cage fusion used in the anterior discectomy for cervical degenerative disc disease. J Spinal Disord Tech 2005;18:326-31. [Crossref] [PubMed]
- Furderer S, Schollhuber F, Rompe JD, et al. Effect of design and implantation technique on risk of progressive sintering of various cervical vertebrae cages. Orthopade 2002;31:466-71. [PubMed]
- Marchi L, Abdala N, Oliveira L, et al. Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. J Neurosurg Spine 2013;19:110-8. [Crossref] [PubMed]
- Sandhu HS, Turner S, Kabo JM, et al. Distractive properties of a threaded interbody fusion device. An in vivo model. Spine (Phila Pa 1976) 1996;21:1201-10. [Crossref] [PubMed]
- Eck KR, Bridwell KH, Ungacta FF, et al. Analysis of titanium mesh cages in adults with minimum two-year follow-up. Spine (Phila Pa 1976) 2000;25:2407-15. [Crossref] [PubMed]
- Galbusera F, Schmidt H, Wilke HJ. Lumbar interbody fusion: a parametric investigation of a novel cage design with and without posterior instrumentation. Eur Spine J 2012;21:455-62. [Crossref] [PubMed]
- Schimmel JJ, Poeschmann MS, Horsting PP, et al. PEEK Cages in Lumbar Fusion: Mid-term Clinical Outcome and Radiological Fusion. J Spinal Disord Tech 2012. [Crossref] [PubMed]
- Blumenthal SL, Gill K. Can lumbar spine radiographs accurately determine fusion in postoperative patients? Correlation of routine radiographs with a second surgical look at lumbar fusions. Spine (Phila Pa 1976) 1993;18:1186-9. [Crossref] [PubMed]
- McAfee PC, Boden SD, Brantigan JW, et al. Symposium: a critical discrepancy-a criteria of successful arthrodesis following interbody spinal fusions. Spine (Phila Pa 1976) 2001;26:320-34. [Crossref] [PubMed]
- Grant JP, Oxland TR, Dvorak MF. Mapping the structural properties of the lumbosacral vertebral endplates. Spine (Phila Pa 1976) 2001;26:889-96. [Crossref] [PubMed]
- Kumar A, Kozak JA, Doherty BJ, et al. Interspace distraction and graft subsidence after anterior lumbar fusion with femoral strut allograft. Spine (Phila Pa 1976) 1993;18:2393-400. [Crossref] [PubMed]
- Hasegawa K, Abe M, Washio T, et al. An experimental study on the interface strength between titanium mesh cage and vertebra in reference to vertebral bone mineral density. Spine (Phila Pa 1976) 2001;26:957-63. [Crossref] [PubMed]
- Liuke M, Solovieva S, Lamminen A, et al. Disc degeneration of the lumbar spine in relation to overweight. Int J Obes (Lond) 2005;29:903-8. [Crossref] [PubMed]
- Steffen T, Tsantrizos A, Aebi M. Effect of implant design and endplate preparation on the compressive strength of interbody fusion constructs. Spine (Phila Pa 1976) 2000;25:1077-84. [Crossref] [PubMed]
- Phan K, Mobbs RJ. Systematic reviews and meta-analyses in spine surgery, neurosurgery and orthopedics: guidelines for the surgeon scientist. Journal of Spine Surgery 2015;1:19-27. [PubMed]