Spinal subdural hematoma: a rare case of spinal subdural hematoma complicating routine, minimally invasive lumbar discectomy and decompression and relevant literature review
Case presentation
A 76-year-old presented with a 9-month history of radiating lower extremity pain, left worse than right, with minimal back pain. The symptoms onset acutely after lifting a heavy ice box. He reported intermittent paresthesias in bilateral L4−L5 distributions without any complaint of weakness. Symptoms worsened with extension, upright posture and Valsalva maneuver and improved with forward flexion. He had no prior spine surgery. He had 3 separate trans-foraminal epidural steroid injections containing anesthetic and cortisone directed at the left L3–4 and L4–5 space, the most recent of which was 1 month prior to presentation. With all injections he noted marked improvement during the anesthetic phase but no durable effect. His past medical history was significant for coronary artery disease status post remote stenting and peripheral arterial disease status post remote carotid endarterectomy. Aside from fish oil and daily low dose aspirin, he was not on anticoagulant medications.
On exam, he had 5/5 motor strength in his bilateral lower extremities with no fixed, focal sensory deficits. His lower extremity reflexes were diffusely diminished though equal bilaterally. He had no upper motor neuron signs and coordination exam was unremarkable.
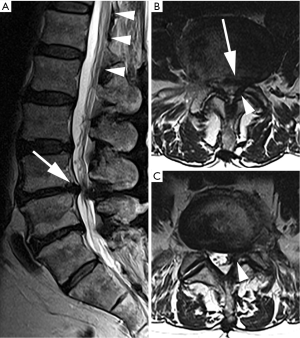
Magnetic resonance imaging (MRI) demonstrated severe central stenosis at L3–4 and left sided lateral recess stenosis at L4–5 secondary to disc herniation with underlying degenerative disc disease (Figure 1). Based on the clinical and imaging findings as well as his failure of a prolonged attempt at non-operative treatment, bilateral L3–4 and left L4–5 partial decompressive laminectomies and L3–4, L4–5 discectomies were recommended. Aspirin and fish oil which were stopped 7 days ahead of surgery.
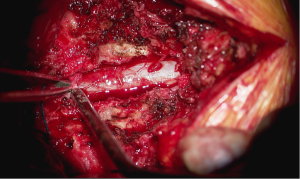
Surgery proceeded without incident. Primary partial decompressive laminectomies via an open midline approach were performed as planned, bilaterally at the L3–4 space and left sided at L4–5. The irrigated burr was used to thin the lamina and Kerrison rongeurs were used to resect the remaining thinned ventral cortex of the lamina to perform the partial laminectomies. Cottonoid patties were placed between the dura and the overlying ligamentum flavum and/or bone to protect against incidental durotomy. Medial facetectomies were performed with the Kerrison rongeur to decompress the lateral recess. The neuroforamen were explored and nerve root decompression was confirmed with a ball probe. The L3–4 and L4–5 discs were then explored. L3–4 was found to have a “hard” calcified disc and no discectomy was performed. At L4–5, there was a combination of “hard” and “soft” disc and the soft extruded nuclear fragments were removed. At the conclusion of the procedure, a Valsalva maneuver was performed to a pressure of 40 mmHg per routine. No active cerebrospinal fluid leak was appreciated nor suspected based on the events of the case.
His immediate postoperative course was uncomplicated, with reported complete resolution of radiating lower extremity symptoms. His neurological examination was normal. He was dismissed from the hospital on postoperative day 1.
However, on postoperative day 2, his wife called the on-call physician to report the patient had developed significant weakness in his lower extremities which had been progressive over the last 12 hours and was rendered unable to get out of bed under his own power. He additionally reported worsening urinary retention over last 12 hours. The patient was taken to the emergency room immediately. On manual motor testing of bilateral lower extremities, his hip flexion and hamstring strength were 4/5, though quadriceps, tibialis anterior, extensor hallucis longus and gastrocnemius/soleus strength was 5/5. His sensory exam was unremarkable. Digital rectal exam demonstrated diminished perianal sensation and weak volitional anal contraction. His post void residual was 800 cc.
Emergent MRI demonstrated normal and expected (1,2) post-laminectomy hematoma at the surgical site, but no severe thecal compression. While the surgical site was unremarkable, cephalad to this was a new eccentric right dorsal subdural fluid collection extending superiorly to the T10–T11 and inferiorly to the L2–L3 discs. This fluid collection was T2 hyperintense and T1 hypointense, suggestive of a subdural seroma or hygroma. It was crowding the cauda equina below the level of the conus medullaris. This fluid collection was noted to enhance internally (i.e., not peripherally) with gadolinium contrast, suggesting intravenous contrast leaked into the space (Figure 2).
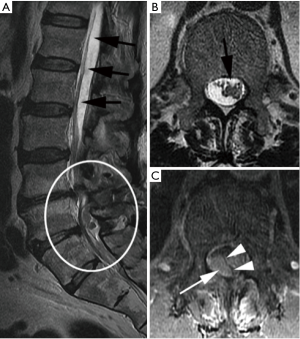
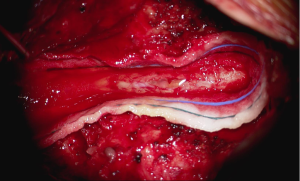
In the setting of acute cauda equina syndrome, the patient was immediately returned to the operating room for exploration and evacuation of the compressive subdural fluid. We first explored the dural tube in the area of the laminectomy defect. Consistent with the emergent MRI, there was no epidural hematoma or other compressive lesion. Likewise there was no durotomy, CSF leak or evidence of infection. We then proceeded with completion laminectomy at L3 as well as central laminectomy of L2 to expose the dural tube at the level of the lower portion of the subdural fluid collection. The thecal sac was noted to be tense and discolored (Figure 3). A midline durotomy was performed. A small amount of xanthrochromatous fluid, (totaling approximately 7–8 cc) was immediately released (Figure 4). This resulted in complete resolution of the subdural fluid collection. The underlying arachnoid was pulsatile, intact and undisturbed (Figure 5). The durotomy was then closed in routine fashion with no CSF leakage appreciated during Valsalva.
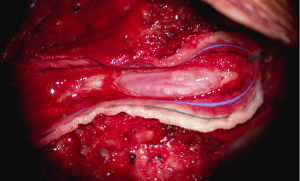
Post-operatively, the patient did very well. His lower extremity weakness resolved and he was independently mobile and no longer requiring intermittent catheterization by postoperative day 2. He was dismissed to subacute rehabilitation for a total of 4 days, and then subsequently dismissed home neurologically intact with complete resolution of his original bilateral lower extremity radiating symptoms. His long-term outcome remained successful with no further complications.
Discussion
The differential diagnosis for pain and progressive neurologic deficit following spine surgery includes compressive phenomenon from intra or extradural sources, infection, infarction or other vascular process (3). Perhaps the most common of these entities is epidural hematoma, with an incidence of less than 0.3% in a review of over 4,000 cases (3). This rate is likely even lower in cases of lumbar decompression in the absence of instrumentation (4). Epidural hematomas have also been reported in sites distant from the operative bed, indicating high index of suspicion is required even in seemingly disparate clinical presentations (5). The imaging of choice for a patient presenting with significant neurologic decline after spine surgery is emergent MRI. However, imaging can be difficult to interpret in the postoperative setting. Sokolowski et al. demonstrated that 58% of all patients develop epidural hematoma of sufficient magnitude to compress the thecal sac beyond its pre-operative state (6). However, the level of compression did not correlate with cauda equina symptoms unless it reached a “critical ratio” which translates to compression of the thecal sac to 20% of its pre-operative volume (2) or an absolute dural sac area of less than 75 mm (7).
Spinal subdural hematoma (SSH) represents an even more rarely encountered explanation for progressive neurologic deficit. It may be spontaneous or secondary to iatrogenic injury. In spontaneous hematoma, traumas, infection, anticoagulation therapy, bleeding disorder, intradural tumor or arteriovenous malformation have been implicated as predisposing conditions (8,9). Iatrogenic injury has also been reported via lumbar puncture, epidural anesthesia or, rarely, direct injury at surgery (10-12).
In the cases of SSH complicating spine surgery, some explanation for dural injury was apparent either with known durotomy (13), or insertion of implants (14,15). These cases were recognized with characteristic findings on emergent MRI imaging after development of progressive neurologic symptoms in the early postoperative period (less than 1 week).
It is critical to distinguish between an epidural and SSH prior to surgical exploration to inform the need for intradural interrogation (16). Axial MRI cuts generally allow for identification of the dura and appreciation of a semi-circular and generally ventral fluid collection, bright on T2 though relatively isointense to CSF in the setting of SSH (17). SSH crowds the cauda equina roots, though perhaps without the significant displacement common to epidural compression, generally most significant dorsal to the thecal sac (18). Enhancement with gadolinium contrast is variably reported (17,19,20), though may be useful in making the radiographic distinction between hygroma and effusion (21).
The most commonly reported treatment of SSH is urgent surgical decompression with durotomy and evacuation of the hematoma (16,22). There are, however, also reports of spontaneously resolving SSH, specifically with limited neurologic deficit and no evidence of progression (22). Postoperative, as opposed to spontaneous, SSH appear to portend a more favorable prognosis in terms of early recognition and neurological recovery (23). Cauda equina as a result of compressive injury to the cord can result in complete recovery with prompt identification and decompression (24,25).
The mechanism of SSH remains elusive. Haines et al have proposed that the subdural space exists as a potential space between the arachnoid and dura which is connected though a lay of border cells with weak, easily sheared intracellular connections. The shearing of the dural layers with minute amounts of trauma disrupts these weak junctions and cleaves open the space we appreciate as the subdural space (26).
Microtears in the arachnoid may permit dissection into the subdural space; however, these represent hygromas with different character and imaging findings (21,27,28). There have been reports of tension hygromas, whereby the tears in the arachnoid function as one-way valves and CSF is pumped into the subdural space with each pulsation but does not diffuse out given the impermeable nature of the cellular junctions of both the arachnoid and the dura (28).
Some authors have proposed that blood products can be traumatically introduced into the subdural by rupturing either a radicular artery accompanying a nerve root or disrupting the epidural plexus of Batson (less likely given its lateral positioning), attracting further fluid by creating an osmotically favorable gradient and negative hydrostatic pressure driving fluid extravasation into this space (19,27,29-31). Bleeding and extravasation of fluid into the subdural space may be exacerbated by alterations in intraabdominal or intrathoracic pressure leading to additional vessel rupture, even in a delayed fashion (22,31). This mechanism may be especially relevant in the setting of the hyperemic inflammatory response in a postsurgical setting and potential for opioid induced constipation in postoperative patients. Additionally, the extravasation of fluid from small, delicate subdural veins explains the contrast enhancement seen in our case and documented in previous reports of SSH (19,20).
In our case, there was no identifiable dural trauma at the time of surgery, confirmed by Valsalva maneuver to 40 mm Hg at the completion of the incident case, per the surgeon’s routine, and reconfirmed with Valsalva and inspection of the dura upon emergent exploration with no noted extrusion of CSF. Certainly, this does not completely exclude dural injury though it is a reassuring sign of an incident free operation. Manipulation of the dura at the time of surgery could conceivably cause shearing and injury to the subdural structures. However, there is no convincing evidence in our case of an injury that accounts for the contrast enhancing SSH, especially since, the dura in question was cephalad to the index surgical site.
While the etiology remains elusive, the potential for a space occupying fluid collection to cause significant neurologic compromise was demonstrated in this case. Vigilance is required for this rare complication even in the setting of routine, minimally invasive surgery. A lack of noted trauma to the dura at the time of surgery does not appear to prevent the development of postoperative SSH. We recommend urgent MRI in the setting of postoperative profound and/or progressive neurologic deficits and emergent surgical decompression in the setting of SSH. Our patient had complete resolution of all deficits within the first 2 days postoperatively, supporting the decision for early surgical intervention. The arachnoid was not opened in our case and no symptomatic recurrence was observed, thus when noted to be intact, we feel that the arachnoid membrane should not be violated at the time of subdural evacuation. Lastly, this case demonstrates that emergent evaluation of a profound and/or progressive neurological deficit in the early postoperative period requires a wide-lens. While most commonly compressive pathology, when present, will be detected at the surgical site, it can occur at any point along the neural axis consistent with the clinical examination findings.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Awwad EE, Smith KR Jr. MRI of marked dural sac compression by surgicel in the immediately postoperative period after uncomplicated lumbar laminectomy. J Comput Assist Tomogr 1999;23:969-75. [Crossref] [PubMed]
- Sokolowski MJ, Garvey TA, Perl J 2nd, et al. Postoperative lumbar epidural hematoma: does size really matter? Spine (Phila Pa 1976) 2008;33:114-9. [Crossref] [PubMed]
- Uribe J, Moza K, Jimenez O, et al. Delayed postoperative spinal epidural hematomas. Spine J 2003;3:125-9. [Crossref] [PubMed]
- Aono H, Ohwada T, Hosono N, et al. Incidence of postoperative symptomatic epidural hematoma in spinal decompression surgery. J Neurosurg Spine 2011;15:202-5. [Crossref] [PubMed]
- Martin CT, Kebaish KM. Postoperative spinal epidural hematoma at a site distant from the main surgical procedure: a case report and review of the literature. Spine J 2010;10:e21-5. [Crossref] [PubMed]
- Sokolowski MJ, Garvey TA, Perl J 2nd, et al. Prospective study of postoperative lumbar epidural hematoma: incidence and risk factors. Spine (Phila Pa 1976) 2008;33:108-13. [Crossref] [PubMed]
- Leonardi MA, Zanetti M, Saupe N, et al. Early postoperative MRI in detecting hematoma and dural compression after lumbar spinal decompression: prospective study of asymptomatic patients in comparison to patients requiring surgical revision. Eur Spine J 2010;19:2216-22. [Crossref] [PubMed]
- Domenicucci M, Ramieri A, Ciappetta P, et al. Nontraumatic acute spinal subdural hematoma: report of five cases and review of the literature. J Neurosurg 1999;91:65-73. [PubMed]
- Chau SY, Tiu SC. Spinal subdural haematoma: a rare complication of low-molecular-weight heparin therapy. Hong Kong Med J 2008;14:64-6. [PubMed]
- Russell NA, Benoit BG. Spinal subdural hematoma. A review. Surg Neurol 1983;20:133-7. [Crossref] [PubMed]
- Jonsson LO, Einarsson P, Olsson GL. Subdural haematoma and spinal anaesthesia. A case report and an incidence study. Anaesthesia 1983;38:144-6. [Crossref] [PubMed]
- Edelson RN, Chernik NL, Posner JB. Spinal subdural hematomas complicating lumbar puncture. Arch Neurol 1974;31:134-7. [Crossref] [PubMed]
- Gehri R, Zanetti M, Boos N. Subacute subdural haematoma complicating lumbar microdiscectomy. J Bone Joint Surg Br 2000;82:1042-5. [Crossref] [PubMed]
- Chang KC, Samartzis D, Luk KD, et al. Acute spinal subdural hematoma complicating lumbar decompressive surgery. Evid Based Spine Care J 2012;3:57-62. [Crossref] [PubMed]
- Chung T, Thien C, Wang YY. A rare cause of postoperative paraplegia in minimally invasive spine surgery. Spine (Phila Pa 1976) 2014;39:E228-30. [Crossref] [PubMed]
- Post MJ, Becerra JL, Madsen PW, et al. Acute spinal subdural hematoma: MR and CT findings with pathologic correlates. Am J Neuroradiol 1994;15:1895-905. [PubMed]
- Küker W, Thiex R, Friese S, et al. Spinal subdural and epidural haematomas: diagnostic and therapeutic aspects in acute and subacute cases. Acta Neurochir (Wien) 2000;142:777-85. [Crossref] [PubMed]
- Yang MS, Tung YW, Yang TH, et al. Spontaneous spinal and intracranial subdural hematoma. J Formos Med Assoc 2009;108:258-61. [Crossref] [PubMed]
- Teksam M, Casey SO, McKinney A, et al. Gadolinium enhancement of spinal subdural collection on magnetic resonance imaging after lumbar puncture. Neuroradiology 2003;45:553-6. [Crossref] [PubMed]
- Warmuth-Metz M, Kühl J, Krauss J, et al. Subdural enhancement on postoperative spinal MRI after resection of posterior cranial fossa tumours. Neuroradiology 2004;46:219-23. [Crossref] [PubMed]
- Mori K, Maeda M. Delayed magnetic resonance imaging with GdD-DTPA differentiates subdural hygroma and subdural effusion. Surg Neurol 2000;53:303-10; discussion 310-1. [Crossref] [PubMed]
- Kulkarni AV, Willinsky RA, Gray T, et al. Serial magnetic resonance imaging findings for a spontaneously resolving spinal subdural hematoma: case report. Neurosurgery 1998;42:398-400; discussion 400-1. [Crossref] [PubMed]
- Börm W, Mohr K, Hassepass U, et al. Spinal hematoma unrelated to previous surgery: analysis of 15 consecutive cases treated in a single institution within a 10-year period. Spine (Phila Pa 1976) 2004;29:E555-61. [Crossref] [PubMed]
- Louwes TM, Ward WH, Lee KH, et al. Combat-related intradural gunshot wound to the thoracic spine: significant improvement and neurologic recovery following bullet removal. Asian Spine J 2015;9:127-32. [Crossref] [PubMed]
- Lee KH, Lin JS, Pallatroni HF, et al. An unusual case of penetrating injury to the spine resulting in cauda equina syndrome: case presentation and a review of the literature. Spine (Phila Pa 1976) 2007;32:E290-3. [Crossref] [PubMed]
- Haines DE, Harkey HL, al-Mefty O. The "subdural" space: a new look at an outdated concept. Neurosurgery 1993;32:111-20. [Crossref] [PubMed]
- Harreld JH, Mohammed N, Goldsberry G, et al. Postoperative intraspinal subdural collections after pediatric posterior fossa tumor resection: incidence, imaging, and clinical features. AJNR Am J Neuroradiol 2015;36:993-9. [Crossref] [PubMed]
- Singleton WG, Ramnarine D, Patel N, et al. Post-operative spinal subdural extra-arachnoid hygroma causing cauda equina compression: a report of two cases. Br J Neurosurg 2012;26:429-31. [Crossref] [PubMed]
- Breuer AC, Tyler HR, Marzewski DJ, et al. Radicular vessels are the most probable source of needle-induced blood in lumbar puncture: significance for the thrombocytopenic cancer patient. Cancer 1982;49:2168-72. [Crossref] [PubMed]
- Masdeu JC, Breuer AC, Schoene WC. Spinal subarachnoid hematomas: clue to a source of bleeding in traumatic lumbar puncture. Neurology 1979;29:872-6. [Crossref] [PubMed]
- Mokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology 2001;56:1746-8. [Crossref] [PubMed]