Three-year follow-up of a randomized controlled trial comparing preoperative neuroscience education for patients undergoing surgery for lumbar radiculopathy
Introduction
Pain, especially acute pain, is a normal human experience and an inability to experience pain poses a significant threat (1,2). Persistent pain and the resultant disability associated with it are not normal and pose a significant challenge for healthcare (3,4). Globally, persistent pain has reached epidemic proportions with the United States (US) alone reporting that more than 100 million people suffer from persistent pain (5-7). Persistent pain is also a concern when it comes to lumbar surgery (LS), where various long-term studies on decompressive LS suggests that 20–25% of post-surgical patients experience persistent pain and disability at 1-year follow-up (8-12).
While pain after LS is to be expected, findings suggest that patients may in fact expect to be “pain free” which questions the current educational models used to prepare patients for such surgery (12-15). Experiencing persistent pain after LS, when little to no pain is expected, may lead to fears that the surgery was not successful and further exacerbate the pain experience (16,17). Louw et al. interviewed patients 4 weeks post-LS and 50% of the patients expressed high levels of fear, given the fact that they were still experiencing pain after their surgery (18).
Recent research has evaluated the use of pain neuroscience education (PNE) in decreasing pain and disability among patients undergoing LS (12,13,19,20). PNE, in direct contrast to traditional anatomical- and Cartesian-based models of pain (1,21), aims to educate patients more about the neurobiological and neurophysiological processes associated with persistent pain, including peripheral and central sensitization, inhibition, facilitation, neuroplasticity, etc. (22-24). Various randomized controlled trials (RCT) and a systematic review have shown that PNE has a positive effect on pain, disability, pain catastrophization, and physical movement for patients with chronic low back pain (CLBP), extending to 1-year outcomes (22-26).
In regard to LS, a preoperative PNE program for LS has been developed (13), tested (19,20) and used in a multi-center RCT with 1-year follow-up (12). The preoperative PNE program for LS has been shown to help patients develop a more realistic expectation regarding pain after LS (14), improved satisfaction after LS, and a 45% reduction in healthcare cost in the one year following surgery (12). These results are quite remarkable, considering a single, 30-minute PNE session resulted in healthcare savings of over $2,000/patient compared to the control group (CG). The purpose of this paper is to report on the outcomes at 3-years postoperatively (12).
Methods
Approval for the original study was obtained from the Health Research Ethics Committee at Stellenbosch University, Cape Town South Africa. Patients with lumbar radiculopathy were recruited from seven clinical sites in the US, based on availability of physical therapists and spine surgeons willing to participate in the study. Once participating surgeons determined that their patient would require LS, their assistants set the surgery date and provided administrative and procedural information to the patients. At the same time, patients were informed about the study examining the effects of a preoperative education program and invited to participate.
If they consented, patients were randomized into one of two groups via computer-generated numbers. They were assigned to a CG which would receive the standard preoperative education and an experimental group (EG) which would receive the same standard preoperative education plus PNE delivered in a one-time 30-minute session with a physical therapist. Patients in the EG were advised that this was their surgeon’s usual practice. Surgeons and their assistants were blinded to group assignments.
All intake forms were completed by the patients without input from medical staff or researchers and placed in pre-paid sealed envelopes, to be mailed to an independent research assistant for data entry. Final data for this part of the study was collected 3 years after each patient’s surgery, via data packets sent out by and returned to the same research assistant.
Study population
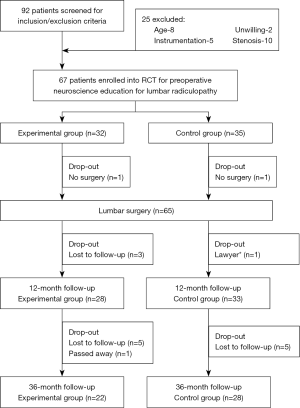
Patients diagnosed with operable lumbar radiculopathy, who were scheduled for LS, were invited to participate. Symptoms had to be predominantly leg pain with or without neurological deficit, and participating surgeons concluded that surgical decompression was warranted. Inclusion criteria were: (I) scheduled for LS for radiculopathy; (II) willingness to comply with the predetermined follow-ups; and (III) willingness to complete postoperative questionnaires at designated time intervals. Exclusion criteria were: (I) younger than 18 or older than 65 years; (II) not being proficient in reading or comprehending the English language; (III) scheduled for LS involving instrumentation (e.g., spinal fusion, arthroplasty); (IV) participation in a formal back school or multi-disciplinary pain management program; (V) undergoing LS for a condition other than lumbar radiculopathy; (VI) presence of chronic pain-related conditions (e.g., fibromyalgia, chronic fatigue syndrome); or (VII) symptoms of cord compression. Ninety-two patients were screened for eligibility and after exclusions, 67 agreed to participate and were enrolled in the study. As noted in the CONSORT (27) study diagram (Figure 1), 65 patients underwent surgery, and of those, 61 completed the initial study with 1-year follow-up. Of these, 11 were lost to follow-up at 3 years, leaving a total of 50 patients.
Trial interventions
Patients in the CG received what constitutes standard preoperative preparation and education from their respective surgeons and staff. To ensure standardization of this education, each participating surgeon (n=7) was asked to complete the Spine Surgery Education Questionnaire (SSEQ) (14). Two investigators independently reviewed the surgeons’ responses to ensure their preoperative education was consistent with the findings of the SSEQ. All participating surgeons provided ‘standard of care’ per the SSEQ.
The development and content of the preoperative PNE has been published elsewhere (13,19,22). Briefly, PNE deemphasizes the traditional anatomical tissue- and Cartesian-based models of pain (1,21), and aims to reduce the fear associated with LBP by providing more information about pain and the neurophysiology of a pain experience. Patients in the EG received the same preoperative preparation and education as the CG with the addition of a preoperative PNE program. The PNE program was provided by participating physical therapists in a one-on-one verbal format, in a conversational and personal approach rather than lecture format and included the use of pictures, examples, metaphors and drawings as needed. Standardization of this PNE program by each therapist was achieved through use of a systematic checklist.
Outcome measures
The primary outcome of interest 3 years after LS was healthcare utilization. Secondary outcome measures were back and leg pain, function and satisfaction ratings regarding the LS.
Healthcare utilization 3-years post LS
Patients were provided with copies of their responses to the 1-year information packets regarding their healthcare utilization in the first year after LS. Patients were then asked to indicate if they had any, and how many, additional of the following medical tests specifically related to their postoperative care: radiographs (X-ray); magnetic resonance imaging (MRI); computerized tomography (CT); bone scan; nerve conduction test (NCT); myelogram; and/or, other medical tests. Additionally, patients were asked to report if they had received any post-surgical treatment or attended for consultations with their spine surgeon; family doctor; physical therapist; other specialist physicians; chiropractor; massage therapist; acupuncturist; psychologist; psychiatrist; and/or, other healthcare professionals. In both cases (medical tests and healthcare providers), patients were asked to indicate how many times they had the tests or treatments. Examples were provided to aid the patients. Data were gathered to determine the total number of visits for each medical test and visits per healthcare provider. For each test and healthcare provider visit, a financial cost was calculated based on the average cost for such tests and visits in the US (www.cms.gov).
Secondary outcome measures
Low back and leg pain were measured using the numeric pain rating scale (NPRS). The NPRS is considered a reliable scaled measure of pain which has a minimal detectable change (MDC) of 2.1 (28), and it has been used in various RCTs for PNE and spinal pain (24,26,29). Level of function at 3-years post-surgery was measured via the Oswestry disability index (ODI) which has very good evidence for its reliability and validity related to low back pain (LBP) (30-32). A change of 5 points (10%) is reported to be the MDC for LBP populations (33). Finally, patients were asked to indicate by means of a numeric scale [1-10] their level of agreement (1 indicating minimal and 10 indicating maximal agreement) with 5 statements about their LS and preoperative educational experience (18,34,35):
- “I am glad I underwent surgery for my leg pain”;
- “I was fully prepared (physically, emotionally, psychologically) for the surgery”;
- “The preoperative education I received prepared me well for the surgery”;
- “Knowing what I know now, I would do this again given the same choices”;
- “The surgery met my expectations”.
Statistical analysis
All analyses were conducted using SPSS version 22.0 (Armonk, NY, USA: IBM Corp) using the significance threshold of α=0.05. As the initial results of this trial (pre, 1 month post, 3 months post, 6 months post, and 1 year post) have already been reported in a previous publication (12), this analysis will focus on the 3-year data. Total medical expenses at 1 year and 3 years after LS were compared for the experimental and CGs using non-parametric Mann-Whitney tests, which was done because of non-normal distribution of the expense data. Medical subgroup expenses (i.e., radiographs, magnetic resonance imaging, computed axial tomography scans, blood work, nerve conduction velocity testing, myelogram, spine surgeon, family doctor, other physician, physical therapy, massage, chiropractic care, acupuncture, psychological services, psychiatry, other) were also analyzed using Mann-Whitney tests.
Mann-Whitney analyses were also used to compare the differences of back surgery-related treatment at the 1- and 3-year points for the following: under current medical care, taking narcotic analgesics, non-steroidal anti-inflammatory medications (NSAIDs), nerve-related medications, antidepressants, and muscle relaxants. The following candidates were analyzed as potential covariates (none met the threshold of correlational coefficients >0.70): age, gender, education, income, and duration of symptoms.
To determine differences in secondary outcome measures between the groups at 3 years, 2 (group: experimental and control) ×2 (time: 1-year post and 3-years post) mixed factorial ANOVAs on three different outcome measures (LBP, leg pain, Oswestry) were conducted. Violations of sphericity were corrected using the Greenhouse-Geisser or Huynh-Feldt methods. Five additional 2 (group: experimental and control) ×2 (time: 1-year post and 3-years post) mixed factorial ANOVAs were conducted for levels of agreement with the 5 statements about LS and preoperative educational experiences (i.e., glad, feeling prepared, prep went well, would do again, met expectations).
Results
Patients
At 3-year follow-up after LS, 50 patients (female: 29; 58%) completed the necessary outcome measures (Figure 1). There were 22 patients in the EG and 28 in the CG, and Table 1 provides descriptive data comparing the initial 65 who underwent surgery to the 50 who completed the 3-year follow-up.
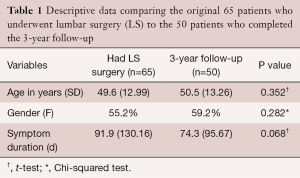
Full table
Healthcare utilization post LS
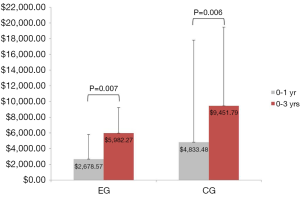
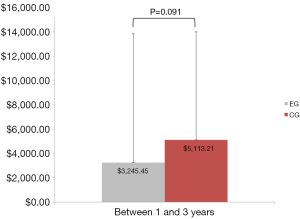
Sixteen of the 50 patients (10 from the EG and 6 from the CG) had no further healthcare utilization (medical expenses related to their LS) from 1- to 3-year follow-up. Additionally, 4 patients (1 from the EG and 3 from the CG) underwent a second surgical procedure between years 3 and 1. As previously reported, overall medical expenses (estimated in U.S. dollars) were 45% lower at postoperative 1 year for the EG (mean =$2,678.57, SD =3,135.30) than they were for the CG (mean =$4,833.48, SD =$3,256.00), Mann-Whitney U=345.00, Z=−2.700, P=0.007. Cumulative medical expenses remained 37% lower at postoperative 3 years for the EG (mean =$5,982.27, SD =12,975.16) than they were for the CG (mean =$9,451.79, SD =$10,005.22), Mann-Whitney U=168.00, Z=−2.736, P=0.006 (Figure 2). Medical expenses between years 1 and 3 were not statistically significant between the two groups, EG (mean =$3,245.45, 95% CI: $−1,453.64 to $7,944.55) compared to the CG (mean =$5,113.21, 95% CI: $1,668.06 to $8,558.37), Mann-Whitney U=223.00, Z=−1.689, P=0.091 (Figure 3).
Over the 3 years after LS, the CG subgroup expenses were significantly higher for family doctor treatment (P=0.019); physical therapy (P=0.002); and massage (P=0.031). None of the other subgroup expenses (i.e., X-ray, MRI, CT, blood work, NCT, myelogram, spine surgeon, other physician, chiropractic care, acupuncture, psychological services, psychiatry, other) were significantly different, ps ≥0.117.
At 3 years after LS, there were no differences between the two groups in those under current care for their back pain (P=0.913) and taking narcotic analgesics (P=0.482); NSAIDs (P=0.642); nerve-related medications (P=0.084); antidepressants (P=0.393); and muscle relaxants (P=0.822).
LBP
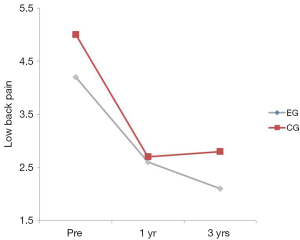
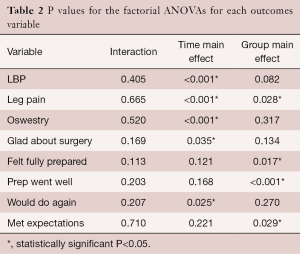
There was no interaction between the EG group and the CG over time on LBP (P=0.405) (Table 2). The group main effect was not significant (P=0.082); however, the main effect of time was, suggesting that both groups improved over time. The bulk of improvement occurred from the pre to the 1 year post-test (P<0.001). No significant differences were found between years 1 and 3 (P=0.068) (Table 2; Figure 4).
Full table
Leg pain
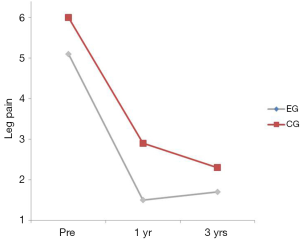
There was no interaction between the EG and the CG on leg pain (P=0.665) (Table 2). The group main effect was significant (P=0.028) with the CG having more leg pain averaged over the duration of the trial. The time main effect was significant suggesting that leg pain decreased over time regardless of group assignment with the only significant improvement occurring between the pre and the 1 year post-test (P<0.001) (Table 2; Figure 5). No significant differences were found between years 1 and 3 (P=0.230).
Low back disability
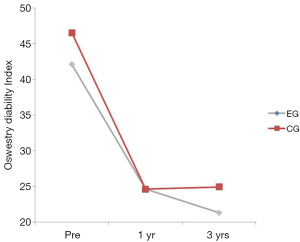
There was no interaction between the EG and the CG on the Oswestry (P=0.520) (Table 2). The group main effect was not significant (P=0.317) but the time main effect was suggesting that low back disability decreased over time regardless of group. Oswestry scores decreased significantly from baseline to 1 year (P<0.001) (Table 2; Figure 6). No significant differences were found between years 1 and 3 (P=0.761).
Post-LS satisfaction
There was no significant interaction between the two groups on post-treatment perceptions of LS (Tables 2,3). The group main effects were significantly different for “felt fully prepared”, “preoperative prepared me well”, and “met expectations” with those in the EG having more agreement with the statements compared to the CG averaged over the 3 years. The time main effect was significantly different for all except “felt fully prepared”, “preoperative prepared me well”, and “met expectations”. Of the two that had a significant main effect for time, the pairwise comparisons did not reveal any change over time for either “glad I underwent surgery” (ps ≥0.058) or “I would do again” (ps ≥0.117).
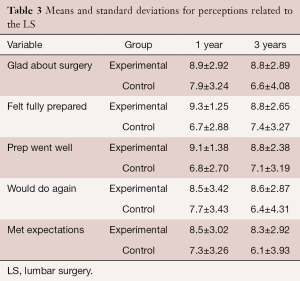
Full table
Discussion
Despite reporting similar LBP, leg pain and disability 3-year after LS, patients who were taught about the neuroscience of pain, continued to spend less on healthcare compared to patients not receiving such education prior to surgery. The results from this 3-year follow-up RCT on preoperative PNE for LS show that favorable behavior changes 1 year after LS persisted 2 years later. Furthermore, this study highlights the relative success of LS for radiculopathy after 3-year regardless of preoperative education, as both groups reported improved LBP, leg pain and disability compared to pre-surgery.
Pain following LS is to be expected and some level of continued pain and disability appears to be the common experience. The results from this study along with various other studies have shown that a percentage of patients who undergo LS for radiculopathy should expect to continue to experience low level LBP averaging of 2.5–3 out of 10 on the NPRS for 6–12 months post-surgery (16,36-39). Similarly, patients following LS also report persistent disability (16,36-39). In this study, three years after LS, patients in both arms of this study reported moderate disability in excess of 20%. Modern pain science, which therapeutically culminates in teaching people about the neurobiological processes of pain, aims to normalize these experiences (22,40). Reconceptualization of pain is a main focus of PNE, along with aiming directly at pain catastrophization and fear-avoidance (22,41-43). This study demonstrates that the introduction of PNE prior to LS led to behavior change in regards to utilization of health care expenditures after surgery. It has been argued that behavior change needs to last 6 months to 5 years to indicate true change (44,45). At 3 years out, this study shows the behavior change found at 1 year continues through the 3 year follow-up.
Traditional models used to educate patients are biomedically driven with information on anatomy, pathoanatomy and biomechanics (40,46,47). It is argued that these models fall short by virtue of their focus on anatomy and pathoanatomy, which cannot readily explain persistent pain beyond the normal expected phases on healing (46). These traditional models can often infer that persistent pain is due to ongoing tissue damage (48,49). In contrast, modern pain science, by embracing neuroplasticity, peripheral and central sensitization as well as facilitation and inhibition provides a plausible understanding of a persistent pain experience (21,40). Evidence of traditional biomedical educational models failing is demonstrated by the study by Morris et al. (50) utilizing a similar multi-center RCT evaluating the outcomes following rehabilitation only, an educational booklet, rehabilitation plus booklet, or usual care only for patients undergoing LS. They found no significant differences in costs or outcomes associated with either intervention (50), while the current preoperative PNE study shows a significant healthcare savings 3-year after LS. The booklet and educational models used by Morris et al. (50) had a primary focus on anatomy, biomechanics and pathoanatomy whereas, the education provided in our trial focused more on pain neurobiology and pain neurophysiology. The biomedical (tissue-based) explanation of persistent pain might well foster a belief that “something is still wrong” (51,52), whereas the PNE approach would explain such pain as a nervous system that has remained ‘sensitive to protect’ (13).
Limitations
This study contains some limitations, which have been highlighted in the published 1-year outcome study (12). For some of the outcomes, the 3-year results were underpowered which was due, in part, to the loss of patients to follow-up.
Conclusions
Adding a single PNE session to patients prior to LS results in significant reduction in healthcare costs 3-year after LS, despite persistent pain and disability. Educating patients about the normal responses to LS in a neuroscience framework may result in significant behavior changes following surgery, and decrease the ongoing healthcare utilization of a large percentage of LS patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Stellenbosch University Board of Institutional Review/Ethics and written informed consent was obtained from all patients.
References
- Moseley GL. A pain neuromatrix approach to patients with chronic pain. Man Ther 2003;8:130-40. [Crossref] [PubMed]
- Melzack R. From the gate to the neuromatrix. Pain 1999.Suppl 6:S121-6. [Crossref] [PubMed]
- Deyo RA, Mirza SK, Turner JA, et al. Overtreating chronic back pain: time to back off? J Am Board Fam Med 2009;22:62-8. [Crossref] [PubMed]
- Orszag PR, Ellis P. The challenge of rising health care costs--a view from the Congressional Budget Office. N Engl J Med 2007;357:1793-5. [Crossref] [PubMed]
- Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Washington (DC): National Academies Press (US); 2011.
- Blyth FM, March LM, Brnabic AJ, et al. Chronic pain in Australia: a prevalence study. Pain 2001;89:127-34. [Crossref] [PubMed]
- Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health 2011;11:770. [Crossref] [PubMed]
- Loupasis GA, Stamos K, Katonis PG, et al. Seven- to 20-year outcome of lumbar discectomy. Spine (Phila Pa 1976) 1999;24:2313-7. [Crossref] [PubMed]
- Yorimitsu E, Chiba K, Toyama Y, et al. Long-term outcomes of standard discectomy for lumbar disc herniation: a follow-up study of more than 10 years. Spine (Phila Pa 1976) 2001;26:652-7. [Crossref] [PubMed]
- Keskimäki I, Seitsalo S, Osterman H, et al. Reoperations after lumbar disc surgery: a population-based study of regional and interspecialty variations. Spine (Phila Pa 1976) 2000;25:1500-8. [Crossref] [PubMed]
- Findlay GF, Hall BI, Musa BS, et al. A 10-year follow-up of the outcome of lumbar microdiscectomy. Spine (Phila Pa 1976) 1998;23:1168-71. [Crossref] [PubMed]
- Louw A, Diener I, Landers MR, et al. Preoperative pain neuroscience education for lumbar radiculopathy: a multicenter randomized controlled trial with 1-year follow-up. Spine (Phila Pa 1976) 2014;39:1449-57. [Crossref] [PubMed]
- Louw A, Butler DS, Diener I, et al. Development of a preoperative neuroscience educational program for patients with lumbar radiculopathy. Am J Phys Med Rehabil 2013;92:446-52. [Crossref] [PubMed]
- Louw A, Butler DS, Diener I, et al. Preoperative education for lumbar radiculopathy: A survey of US spine surgeons. Int J Spine Surg 2012;6:130-9. [Crossref] [PubMed]
- Rönnberg K, Lind B, Zoëga B, et al. Patients' satisfaction with provided care/information and expectations on clinical outcome after lumbar disc herniation surgery. Spine (Phila Pa 1976) 2007;32:256-61. [Crossref] [PubMed]
- Ostelo RW, de Vet HC, Vlaeyen JW, et al. Behavioral graded activity following first-time lumbar disc surgery: 1-year results of a randomized clinical trial. Spine (Phila Pa 1976) 2003;28:1757-65. [Crossref] [PubMed]
- Abbott AD, Tyni-Lenné R, Hedlund R. Early rehabilitation targeting cognition, behavior, and motor function after lumbar fusion: a randomized controlled trial. Spine (Phila Pa 1976) 2010;35:848-57. [Crossref] [PubMed]
- Louw A, Louw Q, Crous LC. Preoperative Education for Lumbar Surgery for Radiculopathy. South African Journal of Physiotherapy 2009;65:3-8.
- Louw A, Diener I, Puentedura EJ. The short term effects of preoperative neuroscience education for lumbar radiculopathy: A case series. Int J Spine Surg 2015;9:11. [Crossref] [PubMed]
- Louw A, Puentedura EJ, Diener I, et al. Preoperative therapeutic neuroscience education for lumbar radiculopathy: a single-case fMRI report. Physiother Theory Pract 2015;31:496-508. [Crossref] [PubMed]
- Melzack R. Pain and the neuromatrix in the brain. J Dent Educ 2001;65:1378-82. [PubMed]
- Louw A, Diener I, Butler DS, et al. The effect of neuroscience education on pain, disability, anxiety, and stress in chronic musculoskeletal pain. Arch Phys Med Rehabil 2011;92:2041-56. [Crossref] [PubMed]
- Moseley GL, Nicholas MK, Hodges PW. A randomized controlled trial of intensive neurophysiology education in chronic low back pain. Clin J Pain 2004;20:324-30. [Crossref] [PubMed]
- Moseley L. Combined physiotherapy and education is efficacious for chronic low back pain. Aust J Physiother 2002;48:297-302. [Crossref] [PubMed]
- Moseley GL. Evidence for a direct relationship between cognitive and physical change during an education intervention in people with chronic low back pain. Eur J Pain 2004;8:39-45. [Crossref] [PubMed]
- Moseley GL. Joining Forces – Combining Cognition-Targeted Motor Control Training with Group or Individual Pain Physiology Education: A Successful Treatment For Chronic Low Back Pain. J Manual Manipulative Ther 2003;11:88-94. [Crossref]
- Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [Crossref] [PubMed]
- Cleland JA, Childs JD, Whitman JM. Psychometric properties of the Neck Disability Index and Numeric Pain Rating Scale in patients with mechanical neck pain. Arch Phys Med Rehabil 2008;89:69-74. [Crossref] [PubMed]
- Moseley GL. Widespread brain activity during an abdominal task markedly reduced after pain physiology education: fMRI evaluation of a single patient with chronic low back pain. Aust J Physiother 2005;51:49-52. [Crossref] [PubMed]
- Deyo RA, Battie M, Beurskens AJ, et al. Outcome measures for low back pain research. A proposal for standardized use. Spine (Phila Pa 1976) 1998;23:2003-13. [Crossref] [PubMed]
- Fritz JM, Irrgang JJ. A comparison of a modified Oswestry Low Back Pain Disability Questionnaire and the Quebec Back Pain Disability Scale. Phys Ther 2001;81:776-88. [PubMed]
- Häkkinen A, Kautiainen H, Järvenpää S, et al. Changes in the total Oswestry Index and its ten items in females and males pre- and post-surgery for lumbar disc herniation: a 1-year follow-up. Eur Spine J 2007;16:347-52. [Crossref] [PubMed]
- Ostelo RW, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976) 2008;33:90-4. [Crossref] [PubMed]
- Toyone T, Tanaka T, Kato D, et al. Patients' expectations and satisfaction in lumbar spine surgery. Spine (Phila Pa 1976) 2005;30:2689-94. [Crossref] [PubMed]
- Yee A, Adjei N, Do J, et al. Do patient expectations of spinal surgery relate to functional outcome? Clin Orthop Relat Res 2008;466:1154-61. [Crossref] [PubMed]
- Dolan P, Greenfield K, Nelson RJ, et al. Can exercise therapy improve the outcome of microdiscectomy? Spine (Phila Pa 1976) 2000;25:1523-32. [Crossref] [PubMed]
- McGregor AH, Dicken B, Jamrozik K. National audit of post-operative management in spinal surgery. BMC Musculoskelet Disord 2006;7:47. [Crossref] [PubMed]
- McGregor AH, Doré CJ, Morris TP, et al. ISSLS prize winner: Function After Spinal Treatment, Exercise, and Rehabilitation (FASTER): a factorial randomized trial to determine whether the functional outcome of spinal surgery can be improved. Spine (Phila Pa 1976) 2011;36:1711-20. [Crossref] [PubMed]
- Ostelo RW, Costa LO, Maher CG, et al. Rehabilitation after lumbar disc surgery. Cochrane Database Syst Rev 2008.CD003007. [PubMed]
- Moseley GL. Reconceptualising pain acording to modern pain sciences. Phys Ther Rev 2007;12:169-78. [Crossref]
- Crabtree JL, Royeen CB, Mu K. The effects of learning through discussion in a course in occupational therapy: a search for deep learning. J Allied Health 2001;30:243-7. [PubMed]
- Wittmann-Price RA, Godshall M. Strategies to promote deep learning in clinical nursing courses. Nurse Educ 2009;34:214-6. [Crossref] [PubMed]
- Oshodi TO. The impact of preoperative education on postoperative pain. Part 1. Br J Nurs 2007;16:706-10. [Crossref] [PubMed]
- Prochaska JO, Velicer WF. Behavior Change: The transtheoretical model of health behaviour change. American Journal of Health Promotion 1998;12:38-48. [Crossref] [PubMed]
- Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot 1997;12:38-48. [Crossref] [PubMed]
- Nijs J, Roussel N, Paul van Wilgen C, et al. Thinking beyond muscles and joints: therapists' and patients' attitudes and beliefs regarding chronic musculoskeletal pain are key to applying effective treatment. Man Ther 2013;18:96-102. [Crossref] [PubMed]
- Louw A, Diener I, Puentedura E. Comparison of Terminology in Patient Education Booklets for Lumbar Surgery. International Journal of Health Sciences 2014;2:47-56.
- Woolf CJ. Central sensitization: uncovering the relation between pain and plasticity. Anesthesiology 2007;106:864-7. [Crossref] [PubMed]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009;10:895-926. [Crossref] [PubMed]
- Morris S, Morris TP, McGregor AH, et al. Function after spinal treatment, exercise, and rehabilitation: cost-effectiveness analysis based on a randomized controlled trial. Spine (Phila Pa 1976) 2011;36:1807-14. [Crossref] [PubMed]
- Greene DL, Appel AJ, Reinert SE, et al. Lumbar disc herniation: evaluation of information on the internet. Spine (Phila Pa 1976) 2005;30:826-9. [Crossref] [PubMed]
- Sloan TJ, Walsh DA. Explanatory and diagnostic labels and perceived prognosis in chronic low back pain. Spine (Phila Pa 1976) 2010;35:E1120-5. [Crossref] [PubMed]
Contributions: (I) Conception and design: A Louw, I Diener, EJ Puentedura; (II) Administrative support: A Louw, K Zimney, EJ Puentedura; (III) Provision of study materials or patients: A Louw, K Zimney, EJ Puentedura; (IV) Collection and assembly of data: A Louw, EJ Puentedura; (V) Data analysis and interpretation: MR Landers, EJ Puentedura; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.